
Pathophysiology of Severe Malaria Infection
- Department of Biochemistry, Imo State University, Owerri, Nigeria
- Department of Biochemistry, Federal University of Technology, Owerri, Nigeria
Abstract
Background: Malaria infection is a multisystem pathology with various clinical complications in both adults and children. The clinical manifestation originates in humans following the invasion of erythrocytes by merozoites.
Methods: The relevant information and data was collated from scientific databases such as Google Scholar, Science Direct, PubMed, Mendeley, Springer Link, and Medline using keywords such as ‘severe malaria infection’, ‘pathophysiology of severe malaria’, ‘complications of severe malaria’ and ‘erythrocyte impairment in severe malaria’.
Results: Generally speaking, the pathophysiology of severe malaria infection encompasses a succession of stages involving the metabolic products of the malaria parasites inclusive of hemoglobin digestion, damaged erythrocyte membrane components, the actions of the pro- and anti-inflammatory cytokines, and the cytoadherence of the malaria parasites to the vascular endothelium as well as sequestration and rosetting. The major complications connected with severe malaria infection include acute respiratory distress syndrome, neurological disorders resulting from cerebral malaria, liver and kidney dysfunction, anaemia and thrombocytopenia, and fatal placental malaria.
Conclusion: The effective management of severe malaria infection involves a proper diagnosis followed by the subjection of the patient to suitable antimalarial treatment with the necessary medications depending on the various clinical manifestations of the infection.
Introduction
Malaria is one of the more widespread health issues worldwide1 caused by the obligate intra-erythrocytic protozoa of the genus , of the following species, specifically , , , and and in addition, recently P. knowlesi infecting humans2. It is noteworthy to mention that the most severe complications and deaths connected with malaria are caused bythespecies Other species, namely , and rarely induce pernicious complications, debilitating relapses, or death3. The incidence of severe malaria is about 2 million cases annually, with approximately 430,000 deaths in the same time period4. A total of 435,000 deaths were reported as a result of malaria globally in 2017, of which 80% of the deaths occurred in Africa. Children under 5 years old are most susceptible to the malaria infection, accounting for 61% of the total deaths from malaria in 20175. Malaria is an emergency pathologic condition that is accompanied by a multisystem disorder with various clinical complications in adults and children6, 7, 8.
Malaria is a life-threatening disease whose clinical manifestation originates in humans following the invasion of erythrocytes by merozoites. After the development of the parasite within the erythrocyte, various waste substances, including the hemozoin pigment and other toxins, accumulate in the infected erythrocyte. These substances are transferred into the vascular system following the lysis of the infected erythrocyte alongside the discharge of the invasive merozoites. There is also the activation of the macrophages and other cells by hemozoin and other malaria parasite toxins. This eventually leads to the generation of cytokines and other soluble factors that play a major role in the initiation of a fever and other pathologies connected with severe malaria9. By implication, the pathophysiology of severe malaria infection encompasses a succession of stages involving the metabolic products of the malaria parasites following hemoglobin digestion, damaged erythrocyte membrane components, the actions of the pro- and anti-inflammatory cytokines, and the cytoadherence of the malaria parasites to the vascular endothelium as well as sequestration and rosetting.
Most complications connected to infections are induced by severe anemia or cerebral malaria. However, varying clinical symptoms also occur based on the parasite species and the organ affected10. Furthermore, the parasite and host-related factors play a major role in the origin and development of severe malaria infections. Infected erythrocytes adhere to the vascular endothelium of various organs, forming rosettes, thereby initiating vascular damage and a host inflammatory/immune response. The differing presentations of thrombocytopenia, acute respiratory distress syndrome (ARDS), and renal and hepatic impairment, including fatal placental malaria, are other complications connected with severe malaria infection10, 11, 12, 13. The present review summarizes the pathophysiology of severe malaria and the varying complications connected with the disease.
Methods
The relevant information and data was collated from scientific databases such as Google Scholar, Science Direct, PubMed, Mendeley, Springer Link, and Medline using keywords such as ‘severe malaria infection’, ‘pathophysiology of severe malaria’, ‘complications of severe malaria’ and ‘erythrocyte impairment in severe malaria’.
Results
Scholarly publications from 1982 - 2020 were chosen from the scientific search engines, resulting in a total of 157 references that have been cited in this review work.
Pathophysiological Processes Involved in the Development of Severe Malaria
Physiological impairment induced by malaria parasites, especially , result in alterations to the normal structure and function of the erythrocytes. This eventually initiates life-threatening complications. In addition, non- infections such as those by and are involved in this pathology and they have been reported to cause severe complications and death in individuals14, 15, 16, 17, 18, 19, 20.
Generally, the pathophysiological events involved are as follows: When the malaria parasites have completed schizogony in the erythrocytes (usually within 24 - 72 hours), the lysis of the infected erythrocytes occurs, leading to the discharge of the merozoite by-products including the damaged erythrocyte membrane components such as glycosylphosphatidylinositol (GPI) and digested hemoglobin– hemozoin pigment. These by-products, especially the GPI and hemozoin, induce the macrophages and endothelial cells to release cytokines and inflammatory mediators like interleukins (IL), namely, IL-1, IL-6, IL-8, lymphotoxin, interferon-γ (IFN-γ), nitric oxide, and superoxide21, 22. The cytokines and membrane products released during the process of erythrocyte lysis have been reported to be accountable for most of the complications that are connected with malaria, such as a fever, headache, weakness, pain in the muscles and joints, diarrhea, central nervous system disorders, stomach discomfort, vomiting, a low blood platelet level, blood clotting impairments, the suppression of the immune system, . (Figure 1)23, 24. Similarly, the DNA of the species is a highly pro-inflammatory agent that can initiate fever and the release of cytokines into the bloodstream. The DNA of species internalized by hemozoin (which is formed in the developmental stage of the parasite in the erythrocytes) undergoes an intracellular interaction with Toll-like receptor-9. This elicits the liberation of pro-inflammatory cytokines and the activation of the cyclooxygenase (COX)-2-mediated upregulation of prostaglandins biosynthesis responsible for the initiation of a fever (Figure 2)25, 26. Hemozoin has also been reported to cause anemia by enhancing the extermination of developing erythrocytes in the bone marrow27, 28.

Sequence of events leading to severe malarial infection (Source
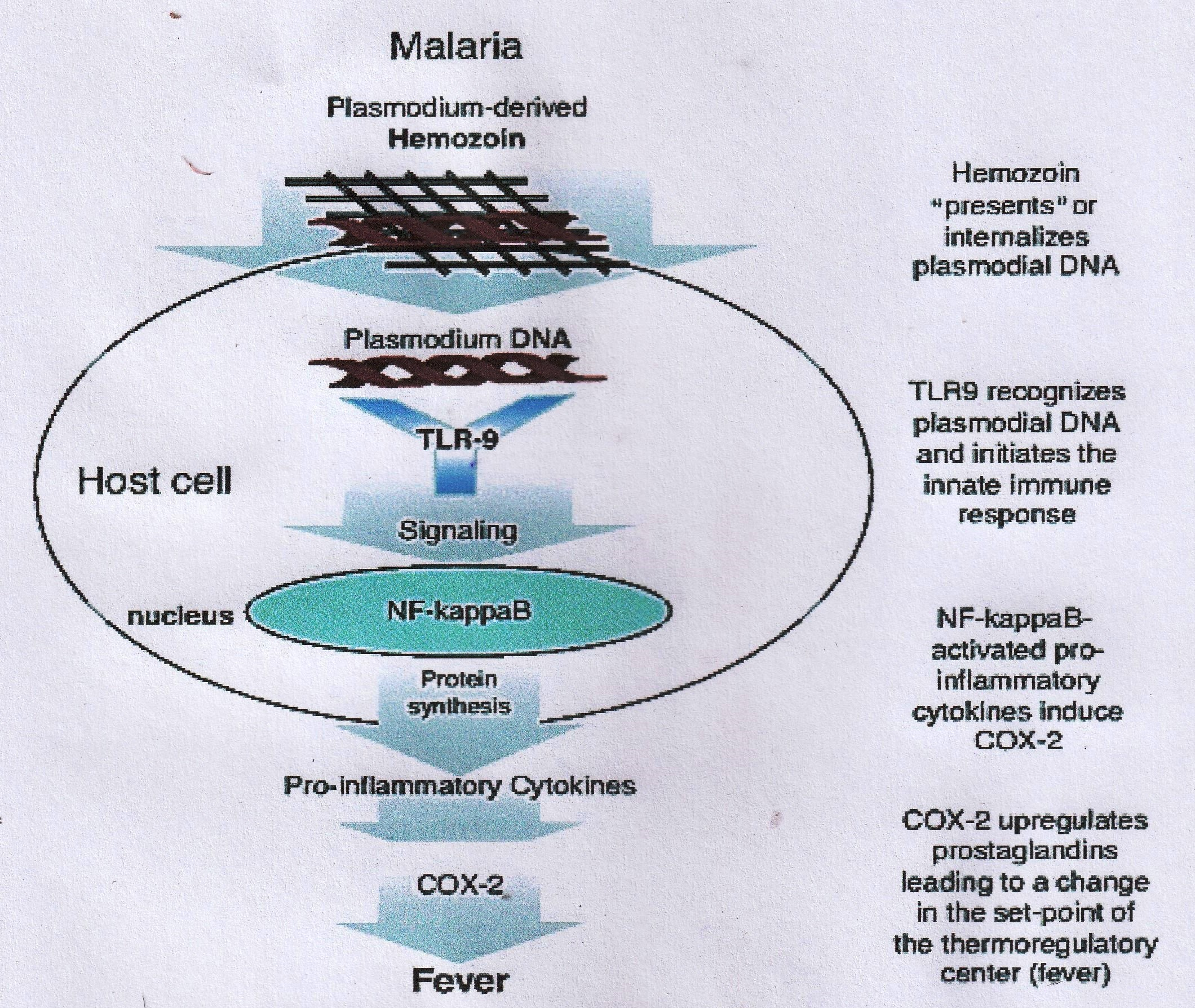
Initiation of fever by the plasmodial DNA (Source:
Role of Cytokines in Severe Malaria
The cytokines as part of the pro-inflammatory cascade such as IFN-γ, ILs, tumor necrosis factor (TNF) and nitric oxide play a foremost role in initiating malaria complications. However, the low plasma levels of cytokines have been reported to ameliorate the virulence of malaria parasites. The failure of the cellular down-regulatory mechanisms in the early stages of the pro-inflammatory cytokine response is responsible for the impairment of the immune system, leading to various connected disorders to severe malaria. Very high cytokine levels have been reported to elicit the following pathophysiology, namely a reduction in the utilization of mitochondrial oxygen, the stimulation of lactate production, the induction of microvascular obstruction, low oxygen levels enhanced by cytoadherence, the obstruction of blood circulation, dyserythropoietic and multifactorial anemia, the inhibition of gluconeogenesis, low glucose levels and myocardial impairment. Additionally, elevated levels of cytokines in the plasma enhance the pro-coagulation process through the stimulation of the leukocytes or platelets, in addition to endothelial injuries as well as vascular damage in the brain and lungs and the upregulation of the cranial and placental vascular and intercellular adhesion molecules (ICAMs) that results in cerebral malaria and drastically affects placental functions21, 22, 26, 29, 30, 31, 32, 33, 34, 35. The equilibrium between pro- and anti-inflammatory cytokines determines the manifestation and severity of the malaria infection22, 30, 32.
Cytokine-mediated injury has also been reported to be connected to the complications encountered in malaria. is known to initiate a higher inflammatory response than due to the greater release of cytokines. infections are depicted by a lower pyrogenic threshold, meaning that they induce a fever at lower parasitaemia levels. The greater pyrogenicity of has been attributed to its structural differences in terms of glycosylphosphatidylinositol and higher levels of Toll-like receptor-9-stimulating motifs in its hemozoin pigment18, 36.

Cytoadherence, sequestration and resetting of P. falciparum infected erythrocytes (Modified from
Cytoadherence and Sequestration
Cytoadherence refers to the efficiency of the parasites to bind to the vascular endothelium10. Mature parasites in the asexual stage and the gametocytes can cling to the vascular endothelium of various organs, including the heart, liver, lung, kidney, and brain, in addition to the placenta and subcutaneous adipose tissues (Figure 3)11, 12.
Sequestration refers to the process whereby infected erythrocytes adhere to the endothelial cells of the capillaries and venules, usually in the late stages of the development of the parasite (trophozoites and schizonts) (Figure 3)38. The sequestration of parasites within the endothelial cells is, for the most part, the pathological basis of severe malaria infection with cerebral malaria inclusive of this39. Parasite sequestration induces local hypoxia through the obstruction of normal blood circulation. Additionally, malaria parasite sequestration stimulates the replication of the parasite and the adhesion of parasitized erythrocytes to the non-infected erythrocytes (a process known as rosetting). Rosetting increases the rigidity of both the normal and infected erythrocytes40. Consequently, malaria parasite sequestration causes the localization of tissue damage that is incurred by the parasite toxins and the activation of the host immune response. This can possibly initiate a focused inflammatory mediator and functional tissue impairment.
Matured forms of the malaria parasites are mostly involved in the sequestration process. This occurs about 20 hours after the erythrocyte invasion. New proteins are synthesized by the parasite which are conveyed to the parasitized erythrocytes' surface, enhancing the infected erythrocyte adhesiveness to the endothelium. There is the possibility of the malaria parasites remaining sequestered in the deep microvasculature for 24 hours during their 48-hour lifecycle. This enables the malaria parasites to elude clearance by the spleen, complicating diagnosis because they can no longer be sighted in the peripheral blood10.
Certain molecules that originate from the parasite and ligands found on the human endothelium have been reported to play a foremost role in the sequestration process of . The major molecules identified to be responsible for this effect include the histidine-rich protein (PfHRP) and the erythrocyte membrane protein 1 (PfEMP1). PfHRP initiates the generation of knobs (symmetric membrane arrangement) on the infected erythrocyte surface. At the same time, PfEMP1 pops out from the knobs and contributes extensively to the sequestration and virulence of the parasite11, 12. The adherence of the parasite to the endothelium occurs in the following order of events. The parasite first adheres loosely to the endothelium, followed by rolling. The parasite then strongly attaches itself to the adhesion molecules of the endothelium (Figure 3). During this process, the sequestration receptor designated as intercellular adhesion molecule-1 (ICAM-1) functions as the rolling receptor, while CD36 ensures stationary and stable adherence under flow41, 42.
Sequestration also occurs in gestational malaria, during which the parasite binds to the placenta. PfEMP1, which is still the major adhesion receptor, binds to the trophoblastic villous endothelium via the chondroitin-4-sulfate and glycosaminoglycans and probably hyaluronic acid (HA). Severe forms of malaria may occur in pregnant women which may lead to fetal death, particularly among women undergoing their first pregnancy. This is because these women have not developed enough of an immunity against the chondroitin-4-sulfate binding parasites43, 44, 45.
Rosetting
Rosetting is a process that involves the cytoadherence of the late stages of an infected erythrocyte to uninfected/parasitized red blood cells (PRBCs) and/or thrombocytes, leading to the formation of erythrocyte rosettes (Figure 3)46. PfEMP1 is the infected erythrocyte ligand that plays a major role in the generation of the rosette. Rosetting takes place through the adhesion of the lectin-like DBL-domain of PfEMP1 present on the infected erythrocyte surface to the complement receptor 1, CD31, and to the heparin sulfate-like glycosaminoglycans of uninfected erythrocytes47, 48, 49, 50. The rate of rosette formation in blood Group O erythrocytes is much less when compared to blood groups A, B, and AB erythrocytes. Individuals that possess blood group O erythrocytes may not suffer from the severe form of malaria in this regard50, 51.
Although , , and have all been reported to be involved in the formation of rosettes, only the rosettes formed by have the capability to initiate severe malaria10, 52. The biomembrane lipid peroxidation product– 4-hydroxynonenal (4-HNE) is probably transported from the infected erythrocyte to the normal erythrocyte in rosettes, thereby enhancing the elimination of the normal erythrocytes by macrophages. This is possibly the cause of the high rate of loss of normal uninfected erythrocytes during severe malarial anemia53.
Rigidity and Deformability of the Erythrocyte Membrane
Distortion in the rigidity and deformability of the erythrocyte membrane plays a foremost role in the emergence of severe malaria complications. In the presence of severe malaria, both the PRBCs and non-PRBCs become rigid37, 54, 55. An increase in the erythrocyte membrane rigidity and a decrease in membrane deformability arises due to the oxidative damage of the erythrocyte membrane engendered by hemin, distortions in the membrane phospholipid bilayer and the attached spectrin network, and thermally driven membrane fluctuations caused by pyrexia, including the blockage of the Na/K pump on erythrocyte membrane. This is caused by nitric oxide22, 55, 56.
A decrease in erythrocyte membrane deformability causes a rise in splenic clearance and a loss of erythrocytes, which leads to anemia. In as much as there is a decrease in erythrocyte membrane deformability in infection, there is an increase in erythrocyte membrane deformability in infection. Although an increase in erythrocyte membrane deformability may enable infected erythrocytes to bypass the splenic sinusoids without being destroyed, the increased fragility of the erythrocytes as a result of the increased erythrocyte deformability can also induce severe anemia in malaria, as well as inhibiting sequestration and enabling the free flow of blood18, 57.
Complications Associated with Severe Malaria Infection
The impairment of the erythrocyte physiochemistry initiates the complications connected with malaria infection through the asexual forms of the malaria parasite. Malaria is a potentially multisystem disease because the erythrocytes that are negatively affected during the malaria infections are needed by all of the organs in the body system32, 58. Fever is a major known symptom of malaria infection, although the illness can advance into the severe malaria form. Even though is the foremost initiator of severe malaria, deleterious health effects and some death cases have been reported to be connected with non- malaria5.
Anaemia
Anemia has been labeled the highest cause of morbidity and death in relation to the malaria infection, especially in children and pregnant women10, 59. Malaria anemia may occur in either an acute or chronic state. The chronic form of malaria anemia is rampant in holo-endemic areas. Acute malaria anemia emanates from the lysis of the erythrocytes caused by increased parasitemia or drug-induced/immune hemolysis60.
The pathophysiological events that result in malaria anemia can be categorized into two groups: the increased damage of PRBCs and non-PRBCs by immune-mediated lysis, phagocytosis, and splenic sequestration in addition to a reduced rate of erythrocyte formation as a result of dyserythropoietic and secondly, the suppression of the bone marrow, the reduced formation of reticulocyte, the actions elicited by inflammatory cytokines and other parasite factors. Furthermore, the reduction in hemoglobin level in malaria patients is induced by co-infection with bacteria, hookworm, and human immune-deficiency virus-1 (HIV-1), poor nutrition, and reoccurring malaria infections in holo-endemic countries59, 60, 61.
The major identified causes of malaria anemia are the increased propensity of PRBCs to rupture and the rapid splenic clearance of distorted PRBCs and non-PRBCs. Non-PRBCs can be prematurely eliminated from the blood by mechanisms involving the rigidity of the membrane, phospholipid asymmetry, and decreased deformability, as previously described40. The action of the spleen has been reported to be a contributory factor in severe malaria by virtue of its capacity to remove PRBCs and non-PRBCs from the systemic circulation excessively. Nevertheless, the spleen can offer protection against severe cerebral malaria62, 63.
Dyserythropoiesis is a major contributor to the pathogenesis of anemia. The phagocytosis of hemozoin by the bone marrow macrophages is known to induce dyserythropoiesis62. Additionally, the immune response plays a principal role in malaria anemia pathogenesis. The monocyte and lymphocyte responses are known to be stimulated by PRBCs, malaria antigens, and hemozoin. In addition, the pro-inflammatory and anti-inflammatory mediators such as IFN-γ, IL-1, IL-23, TNF-α, chemokines, and growth factors generated initiate anaemia60, 64. The macrophage migration inhibiting factor (MIF) is another contributor to severe anemia in malaria infection due to the MIF-induced suppression of bone marrow erythropoiesis65.
Pro-inflammatory cytokines are involved in the iron delocalization pathway of anemia in severe malaria. Ferroportin delocalization is activated by TNF-α. Ferroportin is an essential protein that is found in large amounts in the reticuloendothelial system which mediates the macrophage iron discharge and the absorption of iron in the intestine. Ferroportin delocalization promotes a reduction in iron absorption and macrophage cell release66. Hepcidin is known to stimulate the decreased level of ferroportin in the reticuloendothelial system, and the level of hepcidin is elevated in severe malaria anaemia67.
Micronutrient malnutrition, especially a lack of vitamins A and E, riboflavin, iron, folate, and zinc, has been reported to escalate the complications connected with anemia by disrupting the immune system and iron metabolism dyserythropoiesis59.
Thrombocytopenia
Thrombocytopenia is a common disorder encountered in malaria infection, especially in the early stage of the infection. It is induced by and 8, 68, 69, 70. The occurrence is very prevalent in children and adults. According to the study carried out by Tan . 71 in Thailand, lower platelet counts were reported in pregnant women in comparison to non-pregnant women. The pathogenetic features of malaria thrombocytopenia are vast and may be related to coagulation impairments, splenomegaly and damage to the platelets by macrophages, in addition to the distortion of the bone marrow, oxidative stress, and aggregation of platelets72. Low platelet count and a high level of the von Willebrand factor were reported in and malaria-infected individuals in Indonesia. The High von Willebrand factor is known to be related to platelet binding and therefore can elicit thrombocytopenia73. Another study by de Mast .68 indicated an elevation in the external domain of the platelet receptors for the von Willebrand factor (sGP1b) in the blood of individuals suffering from malaria, thereby inhibiting increased platelet adhesion.
Malaria-associated Acute Respiratory Distress Syndrome (MA-ARDS)
The most genuine complexities seen in intestinal sickness are metabolic acidosis, pulmonary complications, placental malaria, cerebral malaria, and hemorrhages with serious anemia, all of which can prompt ARDS74.
Tragically, there is relatively little epidemiological information on MA-ARDS75. It happens for the most part in grown-ups with fast and helpless anticipation with a lethality pace of 20 — 80%, even with antimalarial treatment76. Manifestations range from gentle respiratory inconveniences, for example, dyspnea and a cough, to an advancement in ARDS75, 76.
In MA-ARDS, most detailed cases are in a low transmission zone or are experienced by non-insusceptible voyagers77. ARDS is connected with intense irritation and damage to the alveolar endothelium and aspiratory parenchyma, which subsequently causes dysfunctions and an expanded porosityof the pneumonic alveolar-capillary hindrance. This promotes the development of edema78. Diminished gas exchange and expanded inflammatory mediators in the lungs bring about respiratory distress in sick patients, prompting death77, 78, 79.
The full intricacy of human MA-ARDS has provoked the necessity to comprehend the physiopathology of the malaria infection76. Accordingly, the murine models have facilitated the understanding of the various intricacies of MA-ARDS pathogenesis, mainly neutrophil inclusion and the levels of hypoxemia like those seen in humans76, 80, 81.
The clinical manifestations identified with MA-ARDS resemble those of ARDS caused by different illnesses in addition to the malaria side effects depicted previously. It is expected to begin with less severe intricacies, for example, a cough and dyspnea advancing until the development of pneumonic edema75, 82. Although the MA-ARDS mechanisms are yet not well established, previous research reports have shown the significance of CD8 T cells in murine MA-ARDS and the activity of leukocytes integrin in the pathogenesis of MA-ARDS83, 84.
Nonetheless, there are two primary events that explain the pathogenesis of MA-ARDS. One is centered on the fiery reaction of the host related to the cellular dysfunction of the pneumonic microvasculature85, 86 and the other is identified by the adhesion of the infected erythrocytes to the endothelial cell of the pulmonary endothelium that promotes the pathogenesis of the infection87, 88. In the lung tissue, the exudative stage is in the beginning stage of the condition where harm to the endothelial hindrance happens because of endothelial cell necrosis, bringing about edema that pours out into the alveoli. This causes the development of a hyaline layer in the alveolar wall80. The histological segments taken from MA-ARDS patients showed bountiful leukocytes, chiefly macrophages in the tissue or alveolar spaces, and a lower number of lymphocytes and neutrophils75, 76. The proliferative fibro stage happens in a later period and it is related to fibroblast cell multiplication and collagen deposition76, 80.
MA-ARDS is associated with the alveoli damage arising from endothelial and epithelial cells injuries, the increment of vascular porousness, and the intercellular spaces followed by edema. During the intra-erythrocyte cycle, the parasite processes hemoglobin to form a compound of the toxic heme group, hemozoin. This prompts the delivery and initiation of pro-inflammatory elements like chemokines, interferon-ɣ (INF-ɣ), CXC-chemokine ligand-10 (CXCL10), CC-chemokine ligand2 (CCL2), keratinocyte-derived chemokine (CXCL1), TNF, IL-1β, IL-6, IL-8, IL-10, transforming growth factor–β (TGF-β) and other inflammatory mediators, for example, heme oxygenase–1 (HO-1)75, 89, 90.
In malaria, PRBCs exhibit a high propensity to adhere to endothelial cells of several organs microvasculature due to the expression of PfEMP191. Proteins intervene in the attachment to non-PRBCs and PRBCs to form rosettes that advance the adhesion to few receptors like CD36, ICAM-1, vascular adhesion molecule-1 (VCAM-1), chondroitin sulfate A (CSA)88, and the endothelial protein C receptor (EPCR)92 present in the endothelial microvasculature of various tissues, for example, cerebral, pneumonic and placental. This attachment permits the parasite to complete its life cycle without being wiped out by hemocatheresis. This exacerbates the severity of the disease88. Notwithstanding, it isn't clear which bond is particularly responsible for the interface between the infected erythrocytes and pneumonic vascular endothelium in MA-ARDS79, 83, 90. Recent research has demonstrated that the development of neutrophil extracellular traps (NETs) is potentially connected to the severity of MA-ARDS and the different intricacies of malaria infection93, 94. Within the blood vessels, NETs shields the endothelial obstruction from the inflammatory components, simultaneously actuating the complement system, influencing hemostasis, designing scattered intravascular coagulation eliciting hemorrhages and thrombi formations, and, subsequently, ischemia94. and experiments performed by Sercundes ., utilizing murine models showed that the treatment needed to restrain the NETs developments resulted in a critical improvement in the MA-ARDS pathology93.
Neurological Complications (Cerebral Malaria)
A major clinical manifestation, which in certain cases leads to death in severe malaria among adults, is cerebral malaria. In the early stage, it is characterized typically by generalized convulsions or drowsiness and confusion, eventually leading to a coma95. From an experimental point of view, changes in the normal mental status of an individual should be handled as though it is cerebral malaria. Furthermore, delirium, agitation, and transient paranoid psychosis can also occur when the patient regains consciousness. Other neurological sequelae different from cerebral malaria, which can also happen in severe malaria, include cranial nerve abnormalities, ataxia, and extrapyramidal tremor2. About 10-50% of the total cerebral malaria cases have been reported to lead to death, even when under treatment96.
Mild stiffness of the neck may occur in this case. Retinal hemorrhages have been observed in about 15% of the total cases investigated. Other common major complications of this condition include fixed jaw closure and tooth grinding (bruxism)5.
Nevertheless, varying disorders such as a dysconjugate gaze, pouting, decerebrate and decorticate rigidity, opisthotonus, vessel changes, plantar reflexes and deep jerks, and papilledema, anemia, hepatosplenomegaly, retinal whitening, and jaundice may possibly occur5, 97, 98.
Liver Pathology in Severe Malaria Infection
The liver is a principal organ that is required during the hepatic phase of the life cycle of the malaria parasite as it is where malaria sporozoites transform into merozoites. The merozoites are delivered into the systemic circulation and enter the erythrocytic stage. In the erythrocytic stage, PRBCs become sequestered in the small blood vessels. The debased haemozoin color is then inundated by nearby tissue macrophages like alveolar and macrophage Kupffer cells. Histopathological observations of the liver in malaria infection incorporate responsive Kupffer cells, the maintenance of the haemozoin color, and insignificant PRBC sequestration99, 100. An ultrastructural study showed there to be a relationship between a high PRBCs load in the liver of malaria patients with jaundice, hepatomegaly and liver enzyme elevation101.
Apoptotic changes occur in various cell systems and include both pathological and physiological changes. While the liver has not been reported to exhibit an apoptotic change in human malaria, these changes have been reported in animal models during the erythrocytic stage in hepatocytes and in the hepatic stage of Kupffer cells102, 103, 104. The process of programmed cell death can intercede in the presence of different enhancers including hormones, growth factors, cytokines, bacterial or viral diseases, and their resistant reactions105. Cell apoptosis is regulated through two significant pathways: the natural or mitochondrial pathway and the extraneous or death-receptor pathway. Initiator caspases, for example, caspase-8 or -9, assumes a regulatory part by enacting downstream effector caspases, for example, due to caspase-3, -6, or -7106. Nuclear factor-kappa B (NF-κB) has been seen to manage the apoptotic program in different cell types, either as an up-controlling reaction or as an apoptosis blocker107. Proof of NF-κB directing apoptosis has been observed in the cerebrum endothelial cells and intravascular lymphocytes in cerebral malaria108.
Likewise, hyperbilirubinemia (> 3 mg/dl) and raised plasma transaminases are diagnostic of malarial hepatitis109. The adhesion of PRBCs to the endothelial walls of the liver vessels prompts the blockage of the intrahepatic ducts, engendering modifications in the bloodstream and as a result, ischemia. Intricacies like hepatic encephalopathy, multi-organ dysfunction, and impaired protein synthesis follow. Histopathological studies have uncovered hepatocyte necrosis, cholestasis, granulomatous sores, and malaria knobs in severe malaria infection110.
As per the World Health Organization (WHO) guidelines, indications of malarial hepatopathy are abnormal in instances of severe malaria infection. Indications of liver dysfunction have been reported in Asia, particularly in India. The majority of these cases are due to malaria or mixed (both and ) malaria111. Cases with adjusted liver capacity tests and surprisingly fulminant hepatic dysfunction have been reported112, 113. An investigation conducted on Nigerian children noted that the elevated plasma levels of liver biomarkers are the biochemical indicators of intense parasitaemia114.
Kidney Pathology in Severe Malaria Infection
Malaria parasites were the main parasitic agents to be plainly connected with glomerular infections in tropical zones. Serious malaria can cause pathology of the glomeruli, tubules, and interstitial locale115. Kidney infection in malaria is principal because of erythrocyte anomalies. PRBCs will, in general, cling to the capillary endothelium, healthy erythrocytes, and blood platelets, prompting the development of rosettes and clusters, which hinders microcirculation116. These pathological events are plausible contributing factors to kidney injury as part of a relationship with hemodynamic instability, including hypovolemia and shock. Endothelial interactions prompt the release of a few cytokines, including thromboxane, catecholamine, endothelin, and other inflammatory mediators that are likewise ensnared in the pathogenesis of malaria-related kidney injury. Invulnerable system actuations in malaria can go through T-cell helper type 1 (Th1) and T-cell helper type 2 (Th2) responses. When the Th2 response prevails in the malaria infection by , complement actuation prompts glomerulonephritis. Hemodynamic unsteadiness occurs because extreme erythrocyte parasitism prompts intense tubular necrosis as observed in infection. At the point when Th1 response prevails, intense interstitial nephritis and intense glomerulonephritis can be observed. Cortical necrosis has additionally been observed in severe malaria infection, describing a more serious kidney injury. For the most part, it is connected with the non-recuperation of renal function, and therefore the advancement of end-stage kidney failure117. A few factors add to the event of these complications, namely vasoconstriction, hypovolemia and hemolysis (prompting hemoglobinuria), erythrocyte parasitemia, resistant edifices deposition in glomeruli, microcirculation dysfunction (due to the cytoadherence of parasitized erythrocytes), and rhabdomyolysis (which isn't normal in malaria). Another contributing factor to kidney failure in malaria is hepatic dysfunction with jaundice and hepatomegaly, through which hyperbilirubinemia can prompt cast nephropathy and acute kidney injury (AKI). Liver dysfunction and its intricacies can likewise cause AKI (hepato-renal disorder)118, 119, 120, 121.
AKI accounts for species ( and ) infections and the patient can deteriorate because of the low hydration and liquid loss brought about by pyrexia spewing, perspiring, and parchedness. Histological examinations have shown that glomerulonephritis, interstitial nephritis, and acute tubular necrosis are evidence of AKI. It is likewise conceivable to discover ongoing kidney dysfunction related to malaria, mostly in the patients experiencing repeated cases of infection116, 117, 122, 123.
Placenta Pathology in Severe Malaria Infection
Malaria in pregnancy is a significant worldwide public health challenge. In 2018, the WHO reported 11 million pregnancies with a high malaria burden in sub-Saharan Africa124. In endemic areas, the incidence of placental malaria can reach up to 63% in pregnant women, regardless of the malaria infection symptomatology125, 126.
Likewise, malaria infection during pregnancy elicits clinical symptoms such as anemia, aspiratory edema, puerperal sepsis, cerebral malaria, hypoglycemia, which can sometimes result in death. In addition, maternal malaria elicits the abortion of pregnancy, intrauterine growth retardation (IUGR), unexpected labor, a low birth weight (LBW), .127
The WHO revealed in 2018 that there were 228 million cases of maternal malaria, of which 93% of such cases occurred in Africa, followed by Southeast Asia (3.4%) and the Eastern Mediterranean Region (2.1%). The prevalence of maternal malaria has been generally steady since 2014 with 57 cases for each 1000 population128.
Placental malaria appears to occur following the evasion of the spleen. The binds to the VAR2-CSA proteins that interact with CSA in the placental intervillous space44, 129. Placental malaria is diagnostic of the presence of parasitized erythrocytes in the intervillous space, invading the maternal monocytes/macrophages130. Noticeable inflammatory actions by the monocytes/macrophages cause enormous, persistent inter-villositis characterized by serious placental malaria131. The inflammatory reactions in placental malaria restrain a vital mechanistic target of rapamycin (mTOR) signaling132. In placental malaria, the autophagy-related genes are downregulated, prompting autophagy dysregulation and in this way, hindered the trans-placental amino acid transport133. Likewise, the blockage of mTOR signaling as a result of placental malaria prompts a diminished placental amino acid uptake134. Recently, it was observed that placental malaria stimulated the placental expression of inflammasomes which were connected to placental discharge and the development of IL-1β, a supportive inflammatory cytokine that engenders a reduced nutrient transporter expression132.
Elevations of pro-inflammatory cytokines, oxidative stress, and apoptosis engender pathological alterations in the placenta135, 136. It has been shown through histopathological alterations that placental malaria exacerbates the danger of toxemia in pregnancy, particularly in primigravidae137. Histopathological alterations during placental malaria showed the presence of hemozoin, perivillous fibrin deposition, syncytial hitch development, and decline in the villous surface area138. These pathologic changes in the placenta may restrict the exchange of nutrients between the mother and embryo, expanding the hazard of limited fetal development and LBW infants139, 140. Placental malaria diminishes the abundance of megalin and disabled homolog 2 (Dab2) in syncytiotrophoblasts, which might be accompanied by LBW141. In Papua, studies showed there to be a relationship between diminished birth weight and placental mitochondrial DNA copy number142. The previous report showed that malaria infection during early pregnancy prompted the modification of the vascular structure of the placenta, diminished transport villi volume, and an increased diffusion distance and diffusion vessel surface. This had an impact on both birth weight and gestational length143. All things being equal, infection in mid-pregnancy is connected with an increased danger of preterm birth, conceivably because of the progressions in the dysregulation of angiogenesis, metabolism and inflammation144.
Maternal HIV infection increases the severity of placental malaria by hindering antibody advancement to the variant surface antigens expressed by malaria-infected erythrocytes while also dysregulating cytokine biosynthesis, and decreasing the defensive IFN-γ reactions145. Reports show that the incidence of malaria diminished in the setting of antiretroviral therapies, especially the administration of protease inhibitors125. Notwithstanding, a new randomized controlled preliminary survey study of HIV-positive pregnant women showed no decline in placental malaria in the presence of a protease inhibitor which was in contrast with non-nucleoside reverse transcriptase inhibitors146. Consequently, antiretrovirals may not assume a defensive role in placental malaria infection.
One investigation showed that infants conceived with placental malaria had higher chances of clinical malaria in their first year of life147. Another survey showed that clinical malaria presents earlier on in the life of infants conceived with placental malaria compared to infants conceived without placental malaria148. A new report established that there were significantly increased chances of the incidence of clinical malaria in neonates conceived by women with placental malaria149. However, a deliberate survey of 14 patients reported a lack of proof when affirming the relationship between malaria in pregnancy and malaria in the earliest stages. There was no obvious proof of vertical transmission150, 151 and the result of innate malaria is uncommon, making the survey outcome inconclusive.
Placenta malaria is described using the sequestration of -infected erythrocytes and invasion of the intervillous spaces of the placenta. The placenta becomes dark because of the deposition of the malaria pigment-hemozoin. The parasite densities are a lot higher in the placenta compared with peripheral blood152, 153. The thickening of the placental basement membrane, perivillous fibrinoid stores, and the syncytial knotting results in an adjusted exchange system between the mother and embryo. The diminished capacity of the placental to deliver nutrients to the fetus causes IUGR154, 155.
Additionally, previous studies have shown that an increased susceptibility to infections during pregnancy results in high parasitemia and a heavy invasion of parasite-tainted RBCs in the placental vasculature44, 156, 157. Besides, it has also been observed that malaria infection is more profound in primigravidae than multigravidae. This protection from malaria infection in multigravidae is because of the improvement of the placental parasite-explicit invulnerability in subsequent pregnancies44, 157.
Conclusions
The major complications connected with severe malaria infection include ARDS, neurological disorders resulting from cerebral malaria, liver and kidney dysfunction, anemia and thrombocytopenia, and fatal placental malaria. However, the effective management of severe malaria infection involves a proper diagnosis followed by the subjection of the patient to suitable antimalarial treatments with the necessary medications depending on the various clinical manifestations of the infection.
Abbreviations
4-HNE: 4-Hydroxynonenal
AKI: Acute kidney injury
ARDS: Acute respiratory distress syndrome
CCL2: CC-chemokine ligand2
COX: Cyclooxygenase
CSA: Chondroitin sulfate A
CXCL1: Keratinocyte-derived chemokine
CXCL10: CXC-chemokine ligand-10
Dab2: Disabled homolog 2
EPCR: Endothelial protein C receptor
GPI: Glycosylphosphatidylinositol
HA: Hyaluronic acid
HIV: Human immune-deficiency virus
HO-1: Heme oxygenase–1
ICAMs: Intercellular adhesion molecules
ICAM-1: Intercellular adhesion molecule-1
IL: Interleukin
IL-1β: Interleukin-1β
IFN-γ: Interferon-ɣ
IUGR: Intra-uterine growth retardation
LBW: Low birth weight
MA-ARDS: Malaria-associated acute respiratory distress syndrome
mTOR: Mechanistic target of rapamycin
MIF: Macrophage migration inhibiting factor
NF-κB: Nuclear factor-kappa B
NETs: Neutrophil extracellular traps
PfEMP1: Plasmodium falciparum erythrocyte membrane protein 1
PfHRP: Plasmodium falciparum histidine-rich protein
PRBCs: Parasitized red blood cells
TGF-β: Transforming growth factor– β
Th1: T-cell helper type 1
Th2: T-cell helper type 2
TNF: Tumoral necrosis factor
VCAM-1: Vascular adhesion molecule-1
WHO: World Health Organization
Acknowledgments
Not applicable.
Author’s contributions
FOO/PCC conceived and designed the scope of the report. FOO/PCC/CMC/CCA/FCL-O contributed in writing the paper. FOO/PCC revised and edited the manuscript draft. CMC/CCA/FCL-O authors were the resource persons who provided all the necessary materials for writing the manuscript. All authors have read and approved the manuscript in the present form and gave the permission to submit the manuscript for publication.
Funding
The authors did not receive financial support and sponsorship from individuals or organizations/institutions.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

