
Fibrinogen Level among Sudanese Patients with Chronic Myeloid Leukemia
- Faculty of Medical Laboratory Sciences, National University, Sudan
- Nahda college, Sudan
- Darfur University College, Sudan
- National Center of Neurological Sciences, Sudan
Abstract
Background: Chronic myeloid leukemia (CML) is a malignancy of white blood cells, distinguished by the Philadelphia chromosome's presence. The biological profile of sufferers with CML at diagnosis is nevertheless missing in Africa, especially in Sudan. Therefore, the study pursued to measure the fibrinogen level among Sudanese patients diagnosed with CML attending the Radiation and Isotopes Centre of Khartoum.
Methods: This descriptive perspective and the analytical casecontrol study were performed at the Radiation and Isotopes Centre of Khartoum from August to September 2021. In this study, fifty patients with CML were utilized as a case, and 50 healthy people were used as a control group. The blood samples from groups were collected in sodium citrate anticoagulant containers. Fibrinogen levels in the blood were determined by the Clauss technique using a coagulometer device.
Results: There was a significant increase in fibrinogen levels between case and control groups. Further, age, gender, family history, and presence of chronic disease were non-significantly variations in CML patients. Moreover, smoking was considered a risk factor in CML.
Conclusions: Fibrinogen level was significantly elevated in CML patients; additionally, there were significant differences associated with smoking, and there were non-significant differences related to age, gender, family history, presence of chronic disorder, and treatment.
Introduction
Chronic myeloid leukemia (CML) is a malignancy of white blood cells; it is a sort of leukemia described by the expansion and out-of-control growth of predominantly myeloid cells within the bone marrow and the gathering of those cells in the blood1. CML was the fundamental malignancy related to clear genetic abnormality, which occurs in chromosomal translocation identified as the Philadelphia chromosome. In this movement, fragments of two chromosomes (9 and 22) transfer locations, a piece of the BCR smash point cluster region from chromosome 22 is fused with the ABL gene on chromosome 9 this uncommon "fusion" gene2.
Fibrinogen is a solvent protein inside the plasma; this is broken down to fibrin through the enzyme thrombin to form clots. The reference range for the extraordinary fibrinogen tests is as follows: fibrinogen antigen 149 – 353 mg/dL, fibrinogen 150 – 400 mg/dL, fibrinogen antigen/functional ratio: 0.59 – 1.23. Regular fibrinogen activity results normally reflect regular blood clotting potential3.
Fibrinogen is an acute-phase reactant, implying that raised fibrinogen levels can be apparent in subsequent situations: infection, tissue damage/trauma, most cancers, acute coronary syndrome, strokes, and inflammatory conditions3, 4.
CML is recognized by a derangement of various parts of the hemostatic system that appear to have thrombo-hemorrhagic complications. Regardless of unusual than different myeloproliferative neoplasms, derangement of different additives of the hemostatic system is seen in CML. Hemostatic abnormalities were described in relation to hyperleucostasis and drugs utilized to treat CML. But, the literature revealed the fibrinogen level is raised in CML5. Therefore, CML patients suffer from many abnormalities that could affect their hemostatic status and might result in bleeding and thrombosis, affecting their survival. So the patients need to frequently check-up for their hemostatic reputation. This study was performed to measure fibrinogen levels in leukemic patients to assist in early detection of any risk that may result in more significant complications, leading to loss of life.
Methods
This is a descriptive prospective, and analytical case-control study carried out at the Radiation and Isotopes center of Khartoum (RICK) from August to September 2021.
Inclusion criteria
Sudanese patients diagnosed with chronic leukemia who attended the Radiation and Isotopes center of Khartoum during the study and accepted to participate in this study were included as a case group and healthful individuals as control with matched age.
Exclusion criteria
Patients with any recently identified hemostatic disease, any family history of bleeding disease, ingesting drugs recognized to affect the hemostatic system, any liver disease, renal disorder, any systemic infection probably to affect hemostasis, pregnant patients, and patients not inclined to present their consent were excluded from the study.
1.8 ml of blood was collected from every member (case & control) in a trisodium citrate container and then obtained the plasma for fibrinogen level determination.
The fibrinogen concentration was measured using a semi-automated coagulometer after dilution of plasma using buffer (thrombin buffer). The coagulometer clot has an optical measurement, which detects a sudden variation in optical density when the clot is formed. A sudden change of optical density activates the chronometer and stirring system. This permits the initiation of time measurement when the sample is added to the reagents and stops measurement time when the clot is formed. The continuous mixing ideally guarantees homogenization, making the measurement possible of low fibrinogen concentration by grouping the fibrinogen filaments in the center of the optical pass. In addition, the system has programmable security time during which variation in optical density, when the reagent and plasma are still in homogenization phase, cannot activate the detection cell.
In this study individually designed questionnaire was used for data collection; SPSS 13.0 statistical program (SPSS Inc., USA) was utilized for statistical analysis (frequency, independent T-test, Anova). The ethical approval to carry out the study was taken from the National University committee, and all information, specimens, and results obtained from patients were considered high-security data.
Results
The epidemiological study
In the current study, 50 CML patients have been incorporated. Among those, 52% had been males, while 48% were females. Furthermore, 50 healthful individuals were selected as the control group; 52% were adult males, while 48% had been female (
Distribution of gender in the study group
|
Study group |
Gender |
Frequency |
Percentage |
|---|---|---|---|
|
Case |
Male |
24 |
48.0 |
|
Female |
26 |
52.0 | |
|
Total |
50 |
100.0 | |
|
Control |
Male |
24 |
48.0 |
|
Female |
26 |
52.0 | |
|
Total |
50 |
100.0 |
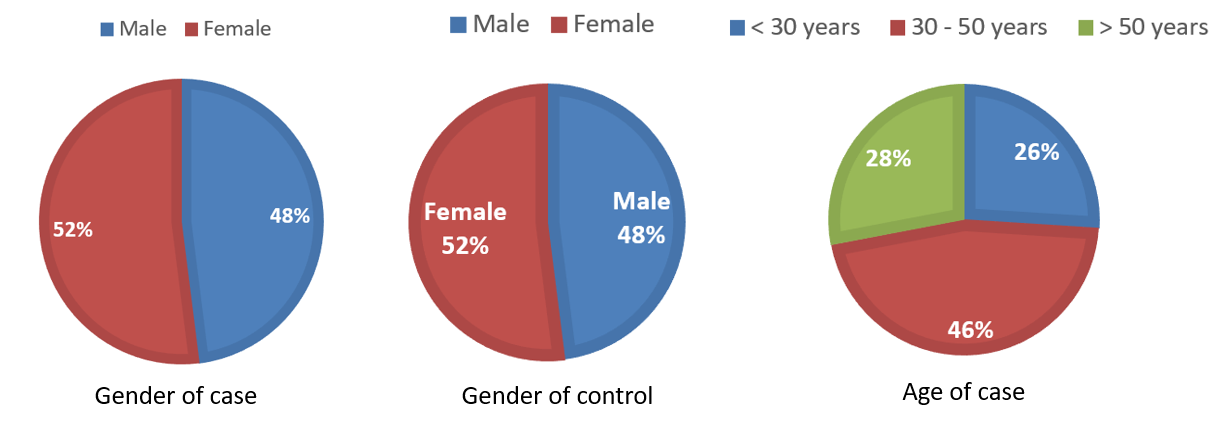
Distribution of gender in case and control groups, and the age in the case group.
Age groups of affected patients
|
Age |
Frequency |
Percentage |
|---|---|---|
|
< 30 years |
13 |
26.0 |
|
30 - 50 years |
23 |
46.0 |
|
> 50 years |
14 |
28.0 |
|
Total |
50 |
100.0 |
Distribution of other studied variables in the case group
|
Variables |
Frequency |
Percentage | |
|---|---|---|---|
|
Family history |
Yes |
15 |
30.0 |
|
No |
35 |
70.0 | |
|
Total |
50 |
100.0 | |
|
Smoking |
Yes |
18 |
36.0 |
|
No |
32 |
64.0 | |
|
Total |
50 |
100.0 | |
|
Alcohol |
No |
50 |
100.0 |
|
DM |
Yes |
25 |
50.0 |
|
No |
25 |
50.0 | |
|
Total |
50 |
100.0 | |
|
Hypertension |
Yes |
17 |
34.0 |
|
No |
33 |
66.0 | |
|
Total |
50 |
100.0 | |
|
Autoimmune |
Yes |
7 |
14.0 |
|
No |
43 |
86.0 | |
|
Total |
50 |
100.0 | |
|
Treatment |
Chemotherapy |
31 |
62.0 |
|
Radiotherapy |
19 |
38.0 | |
|
Total |
50 |
100.0 |
The hematological study
When compared the fibrinogen level between cases and control group there was significant differences with (P = 0.000) (
Comparison of fibrinogen level among the study group
|
Variables |
Minimum |
Maximum |
Mean |
Std. Deviation |
P-value | |
|---|---|---|---|---|---|---|
|
Case | ||||||
|
Fibrinogen (mg/dl) |
50 |
204 |
402 |
288.5 |
44.2 | |
|
Control | ||||||
|
Fibrinogen (mg/dl) |
50 |
163 |
227 |
192.8 |
19.9 | |
|
0.000* | ||||||

Mean of fibrinogen among the study group.
Comparison of fibrinogen level among other studied variables in the case group
|
Variables |
Fibrinogen (mg/dl) |
P-value | |
|---|---|---|---|
|
Gender |
Male (n = 24) |
286.4 ± 46.4 |
0.734 |
|
Female (n = 26) |
290.5 ± 42.9 | ||
|
Family history |
Yes (n = 15) |
289.1 ± 50.7 |
0.951 |
|
No (n = 35) |
288.3 ± 41.9 | ||
|
Smoking |
Yes (n = 18) |
272.2 ± 39.2 |
0.049* |
|
No (n = 32) |
297.8 ± 44.8 | ||
|
DM |
Yes (n = 25) |
288.9 ± 37.6 |
0.952 |
|
No (n = 25) |
288.2 ± 50.8 | ||
|
Hypertension |
Yes (n = 17) |
293.5 ± 32.8 |
0.577 |
|
No (n = 33) |
286.0 ± 49.4 | ||
|
Autoimmune |
Yes (n = 7) |
309.6 ± 36.5 |
0.178 |
|
No (n = 43) |
285.1 ± 44.8 | ||
|
Treatment |
Chemotherapy (n = 31) |
284.1 ± 46.2 |
0.373 |
|
Radiotherapy (n = 19) |
295.7 ± 41.1 |
Comparison of fibrinogen level among other studied variables in the case group
|
Variables |
Fibrinogen (mg/dl) |
P-value | |
|---|---|---|---|
|
Gender |
Male (n = 24) |
286.4 ± 46.4 |
0.734 |
|
Female (n = 26) |
290.5 ± 42.9 | ||
|
Family history |
Yes (n = 15) |
289.1 ± 50.7 |
0.951 |
|
No (n = 35) |
288.3 ± 41.9 | ||
|
Smoking |
Yes (n = 18) |
272.2 ± 39.2 |
0.049* |
|
No (n = 32) |
297.8 ± 44.8 | ||
|
DM |
Yes (n = 25) |
288.9 ± 37.6 |
0.952 |
|
No (n = 25) |
288.2 ± 50.8 | ||
|
Hypertension |
Yes (n = 17) |
293.5 ± 32.8 |
0.577 |
|
No (n = 33) |
286.0 ± 49.4 | ||
|
Autoimmune |
Yes (n = 7) |
309.6 ± 36.5 |
0.178 |
|
No (n = 43) |
285.1 ± 44.8 | ||
|
Treatment |
Chemotherapy (n = 31) |
284.1 ± 46.2 |
0.373 |
|
Radiotherapy (n = 19) |
295.7 ± 41.1 |
Discussion
This study was carried out at the Radiation and Isotopes center of Khartoum (RICK) from August to September 2021 to measure fibrinogen levels in CML patients. The collected data were analyzed using the statistical package of the social science (SPSS) program. There was new vision revealed using this study showed that the male: female ratio was 1.1: 1. This finding supports AnKur . study, which found out that in CML, males are influenced more frequently than females (ratio of 1.3 – 2.2 to 1) because women have protection because of increased expression of tumor suppressor genes6, 7
This study indicated that the most affected age group was 30 – 50 years, that's constant with the different study which mentioned the prevalence of CML will increase with age8.
When compared the fibrinogen level among cases and controls groups, there was a highly significant rise in the fibrinogen level of cases. This result is consistent with Evica ., which determined that fibrinogen levels are increased in 36.27% of the CML patients9. Abegunde also inferred that increased fibrinogen levels are a risk factor for thrombosis in these patients10. This hyperfibrinogenemia may be explained because the growth of tumor cells impacts homeostasis by activating the coagulation cascade, which promotes thrombin formation11. Moreover, tumors cells typically secret chemokines, cytokines, and prostaglandins that enhance the recruitment of inflammatory cells resulting in an increase of fibrinogen level as an acute-phase protein12.
In the current study, there was a non-significant difference in fibrinogen level in age (years) (p-value ≥ 0.05), gender (p-value ≥ 0.734), and family history (p-value = 0.951) of CML patients. On the other hand, there was a significant difference in fibrinogen level in smoker CML patients (p-value = 0.049). Many research emphasized that smoking is the most vital and most common risk issue in youth patients13.
Similarly, there was a non-significant difference in fibrinogen level when compared among the patients with different chronic diseases such as DM (p-value = 0.952), HT (p-value = 0.577), and autoimmune disease (p-value = 0.178) which result agreed with study showed that diabetes and HTN isn't any independent predictor of hyper-fibrinogenemia14.
Finally, there was no significant difference in fibrinogen level when compared between the patients treated by chemotherapy and patients treated by radiotherapy (p-value = 0.373). This finding agreed with the previous study that revealed that blood fibrinogen concentration decreases in patients with primary tumors after chemotherapy15.
Conclusions
This study showed that the fibrinogen levels in blood were significantly raised in the CML patients. In addition, there were significant differences associated with smoking. On the other hand, there were non-significant differences in age, gender, family history, presence of chronic disorder, and treatment. To adopt this result study with larger samples and a more extended period should be conducted to evaluate coagulation and fibrinolysis parameters which might have varying results than the present study results.
Abbreviations
CML: Chronic myeloid leukemia
DM: Diabetes Millets
HT: Hyper Tension
RICK: Radiation and Isotopes center of Khartoum
SPSS: Statistical Package Of Social Science
Acknowledgments
The authors acknowledge the personnel of the Radiation and Isotopes center of Khartoum, Sudan for their beneficial and support.
Author’s contributions
All composer similarly participated to this work , wrote, rectified and authorized this manuscript. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

