
An Overview of Cytokine Trafficking during COVID-19 Infection
- Department of Zoology, University of Okara, Renala Khurd,56300, Pakistan
- Applied Molecular Biology and Biomedicine Lab, Department of Zoology, University of Narowal, Narowal, Pakistan
- Cell &Molecular Biology Lab, Institute of Zoology,University of the Punjab, Lahore-Pakistan
Abstract
The end of the first quarter of the 21st century will be known as when there was a deadly pandemic due to COVID-19 infection. The four subfamilies of a, b , △, g have a genetic variation ranging between 26 to 32 kb. Patients with COVID-19 have more than 140 inflammatory cytokines activated and this relates to the disease severity, progression, and the hyperactivation of T cells. In viral diseases, an abnormal pro-inflammatory factor release damages the lung physiology and causes oxygen deprivation. This is primed by the unregulated fabrication of a high risk of inflammatory factors like interleukin members, as well as also increasing the level of CRS and chemokines. Cytokines activate the JAK-STAT and Ras-MAPK pathways and stimulate the CRP value, the clear marker used to show infection in the body. On the other hand, for cytokines, an interleukin member is required for lymphocyte growth and development. Treatments which have been shown to effectively reestablish lymphocyte count in a variety of viral infections have been safely delivered to septic shock patients with lymphocyte abnormalities comparable to those seen in COVID-19. Many therapies have been approved and some are under trial for the effective treatment of viral infections including Tocilizumab and Siltuximab.
Introduction
Coronaviruses are enclosed, single-stranded massive RNA viruses with a positive single-stranded RNA strand in living organisms1. Coronaviruses are subdivided into four subfamilies; α, β, δ, and Δ. While α and β coronaviruses are assumed to have originated in mammals like bats, the δ and Δ viruses are primarily found in some mammals and aves specie. The genome size ranges from 26 to 32 kb. Among these subfamilies, more than six coronavirus subtypes are able to infect humans. Beta coronaviruses can potentially cause illness and death, while alpha coronaviruses generate asymptomatic or mildly indicative infections. SARS-CoV-2 is a beta coronavirus of the B lineage that is usually correlated to the SARS-CoV virus1, 2.
The key four viral genomes transcribe diverse N, S, SMP, and M, with HKU1 beta coronaviruses exposing a transitory membrane glycoprotein (HE)3. Pneumonia was an early diagnostic sign of the SARS-CoV-2-accompanying infection COVID-19, according to Chan JF and his colleagues. GI symptoms and asymptomatic infections have also been reported in modern investigations, especially in younger nurslings4. SARS-CoV-2, like many other viruses, infects the alveolar epithelial cells in the lungs through receptor-mediated endocytosis with the ACE2 serving as an entrance receptor2.
COVID-19 spreads through a systemic inflammation triggered by immune system hyperactivity in reaction to the virus infection. Lung tissue loss, pulmonary-edema fluid exudation, dyspnea, and pulmonary illness can develop from this persistent inflammation5. When compared to healthy lungs, the coronavirus produces a significant decrease in alveolar lacunar space, enhanced immune infiltration, and cell death through apoptosis6. COVID-19 decreases the number of lymphocytes in the peripheral blood while elevating the provocative cytokine concentration in the serum. In extreme COVID-19 cases, cytokines, which are most presumably produced by inflammatory monocytes, may be responsible for considerable lung inflammation and pulmonary function deterioration7.
In the case of COVID-19, these inflammatory cytokine markers help to diagnose the disease progression and sternness8. A cytokine storm is an immunological state marked by prompt propagation and the hyperactivation of T cells (white blood cells), macrophages, natural killer cells (NK cells), and increased production of more than 140 inflammatory markers with biochemical mediators secreted by immune and non-immune cells9, 10. In viral diseases, an abnormal pro-inflammatory factor release damages the lung physiology and the epithelial cell barrier of the alveoli resulting in vascular discharges, edema, and oxygen deprivation which leads to the irregulated fabrication of pro-inflammatory factors known as IL-6, 8, 1, GM-CSF, as well as chemokines11. In the current study, the role of interleukin members in COVID-19 has been evaluated.

Pathogenicity of COVID-19 and role of IL-6.SARS-CoV-2 mainly penetrates the nostrils, eye, and mouth when enters the host cell via an add-on to their receptors which induces an inflammatory effect. ACE2: angiotensin-converting enzyme, LT: leukotriene, ROS: reactive oxygen spices, CRP: c-reactive protein, TNF-α: tumor necrosis factor-alpha, IFN-I: interferon type I, IL-6: interleukin6, IL-6R: interleukin-6 receptor, ARDS: acute respiratory distress syndrome.
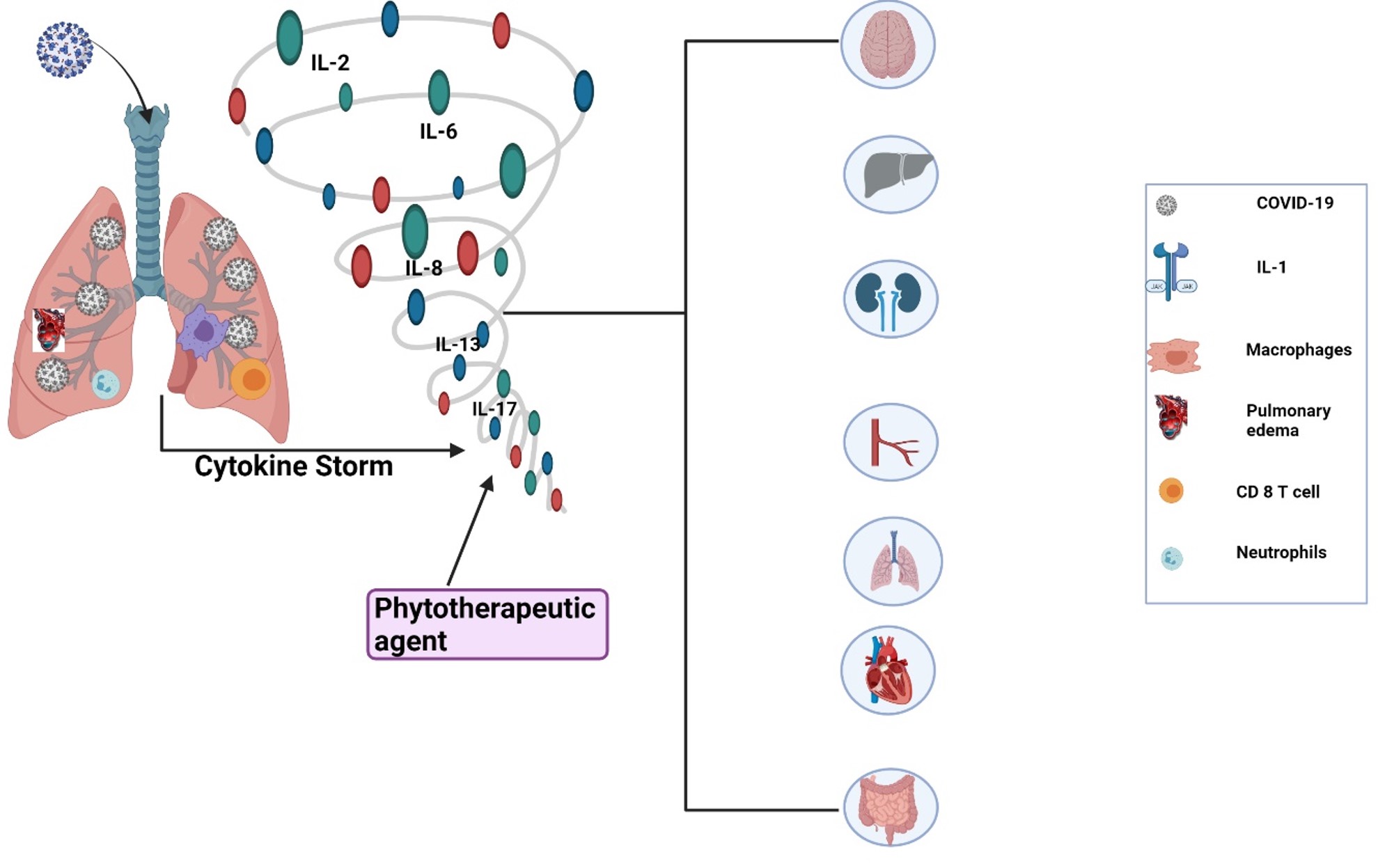
The spectrum of the destruction of different body organs by cytokine storm (interleukin family) produced during COVID-19.
Role of interleukin-6 (IL-6)
Multiple roles are served by IL-6 and it was initially discovered as a B-cell developmental factor involved in the maturation of immune response cells. Since then, it has been shown that IL-6 has a diverse variety of potential functions, including effects on T cells, blood vessels, and neurons12.
During the acute phase of viral infection, there is a systemic amplification of IL-6 and it has pleiotropic protein that is generated and comes back to tissue trauma and microbial infections. Fibroblasts, keratinocytes (skin cells), mesangial cells, vascular endothelial cells, mast cells (also known as mastocytes), macrophages, dendritic cells (nerve cells), and T and B cells (WBCs) are among the cell types that produce cytokine. In COVID-19, this leads to the patient's lungs being destroyed13, 14, 15.
Several studies have found that IL-6 is significant in the immune-pathogenesis of COVID-19, as indicated by the distinguished blood concentration of this cytokine, particularly in extreme situations16, 17, 18, 19. Huang . reported higher IL-6 echelons in the case of COVID-19. It interacts through a polypeptide chain called the IL-6 receptor (IL-6R), which subsequently binds to a membrane glycoprotein called gp130 to activate intracellular signaling via the JAK-STAT and Ras-MAPK pathways18. In the body, like the central compartment and excretory products such as urine, a soluble derivative of IL-6R (sIL-6 R) is also present. sIL-6 R is generated from a disinterring and metalloprotease17 degradation of IL-6 R20. The activation of IL-6 channels promotes the liver cells to generate and secrete an acute and C-reactive protein (CRP) such as serum amyloid A, fibrinogen, and haptoglobin, which is a glycoprotein complex produced in the liver, and 1-antichymotrypsin while diminishing fibronectin, albumin, and transferrin development21. COVID-19 is a condition in which IL-6 plays a critical role since it is implicated in the disease etiology and is clinically correlated with prognosis22. Particularly, IL-6 has been shown to be a potential biological indicator in a multitude of infections, including pneumonia of various etiologies. It is frequently used in clinical practice and research21, 23, 24. Chen . reported that an enhanced baseline IL-6 was associated with physiological parameters and the finding of serum SARS-CoV-2 RNAemia which appears to be diagnostic for critical illness. After a comparison of severe patients, it was observed that critically ill patients had about 10-fold higher IL-6 levels and all fatal cases had incredibly high IL-6 levels25. The increased starting point of a further provocative surrogate marker, including CRP, lactate dehydrogenase (LDH), ferritin, and D-dimer, as well as chest computed tomography (CT) abnormalities, were also determined to be favorably linked with IL-6. Patients healing from COVID-19 had lower IL-6 levels and improved lung physiology but the IL-6 levels elevated where there was an illness re-exacerbation26. Pandolfi et al. stated that in the case of COVID-19, the patients admitted to the ICU had higher IL-6 levels than the other admitted people in poor health27. In individuals with SARS-COV-2 infection, IL-6 has a potentially harmful function28. Additionally, certain cytokines can induce significant pulmonary destruction by aggregating neutrophils and macrophages in the respiratory tract, resulting in the formation of hyaline membranes and drawn-out concealing of the alveolar barriers, as well as tubule-interstitial destruction29.
CRS is defined as an abnormal inflammatory reaction with clinical manifestations varying from a flu-like syndrome to an unregulated systemic inflammatory response and multi-organ disturbance, and it is connected with macrophage activation, T lymphocyte modulation, and endothelial cell amplification, as well as increased inflammatory cytokine production. IL-6 is linked to cardiomyopathy, the hastening of complement and coagulation pathways, disseminated intravascular coagulation, and vascular leakage among some of the discharged cytokines. CRS is most common in people who have received various types of immunotherapy or haploidentical allogeneic hematopoietic cell transplantation; nonetheless, COVID-19 patients may develop a CRS-like condition30, 31.
The deadly consequences of COVID-19 may be due to increased cytokine levels. Various putative therapeutics targeting the host immune system, such as inflammatory cytokine inhibition, stem cell therapy, immune cell reduction, postpartum plasma transfusion, and false extracorporeal liver sustenance, may be beneficial in the treatment of COVID-1932. The blocking of IL-6 is a potential therapy used for COVID-induced CRS because its level is already reported in COVID-19 cases33. As a result, targeting IL-6 for COVID-induced CRS could be beneficial as shown in
Table shows the IL-6 Blockers
|
Disorders |
IL-6 Inhibitors |
Results ( approved indications) | |
|---|---|---|---|
|
Castleman |
Tocilizumab |
There was upgrading in inflammatory symptoms and a reduction in steroid dose without an increase in adverse events | |
|
Siltuximab |
Development in provocative indicators increases the frequency of adverse events in patients with multicentric Castleman disease | ||
|
Systemic juvenile idiopathic arthritis |
Tocilizumab |
In individuals with active sJIA, there was a greater improvement in signs and symptoms when compared to placebo, as well as with catch-up growth | |
|
Adult-onset Still’s disease |
Tocilizumab |
Improvement in CR in glucocorticoid-resistant AOSD patients without attaining a statistically significant difference compared to placebo; glucocorticoid dose reduction | |
|
Rheumatoid arthritis |
Tocilizumab |
Recovery in symptoms and signs in active RA patients without such an increase in the incidence of disease | |
|
Cytokine release syndrome |
Tocilizumab |
CAR T-cell treatments cause CR in CRS | |
|
Takayasu arteritis |
Tocilizumab japan |
Upgrading in time to deterioration in Tocilizumab group paralleled to placebo | |
|
Giant cell arteritis |
Tocilizumab |
Patients with a specific analysis or recurrence of large cell arthritis have a stronger CR | |
|
| |||
|
RA |
Sirukumab |
In individuals with active RA, there was an enhancement in warning sign severity, damage in structural progression, and quality of life | |
|
Psoriatic arthritis |
Clazakizumab |
Compared to placebo, there was an improvement in musculoskeletal symptoms, but not in skin disease | |
|
Systemic sclerosis |
Tocilizumab |
Tocilizumab reduced required energy capacity decline when compared to placebo; no alteration in skin solidifying decrease | |
|
Severe Viral Infections |
Sarilumab |
Sarilumab is a monoclonal antibody that targets together IL-6R and mIL-6R and is entirely human | |
|
| |||
|
Mycophenolate mofetil |
MAS and HLH |
Hang-up of inosine monophosphate dehydrogenase |
Yes |
|
HSC transplantation |
Hemophagocytic lymphohistiocytic |
Spare by hereditarily normal bone marrow |
Yes |
|
Cyclosporine A |
Widely used for primary and secondary HLH |
Inhibiting NF-AT migration into the nucleus to reduce the function of overactivated T cells |
Yes |
|
Corticosteroids |
Increased levels of cytokines |
Additional with inherently regular bone marrow |
Yes |
|
Siltuximab |
“CRS” |
The anti-IL-6 antibody |
Yes |
|
Aspirin |
Acute lung injury and ARDs |
Antiplatelet effect to diminish the polymorphonuclear (PMN)leukocytes conscription |
Yes |
|
Anakinra |
MAS, sepsis, HIV/AIDS-associatedHLH, andCRS |
IL-1 receptor antagonist blockingIL-1α andIL-1β |
Yes |
|
Rilonacep |
MAS |
Counterbalancing of IL-1α and IL-1β |
Yes |
|
Tadekinigalfa |
NLRC4-associatedMAS |
Human IL-18-binding protein recombinant |
Yes |
Interleukin-7 (IL-7)
Interleukin-7 (IL-7) was discovered more than a decade ago55. The human IL-7 gene was detected on chromosome 8q12-1356. IL-7 is a cytokine secreted by stromal cells in the lymphoid organs which are essential for T cell growth and survival in the periphery. Exogenous stimulation has little effect on IL-7 secretion by the stromal cells, unlike most other cytokines that act on lymphocytes57. This pleiotropic or multiple effected interleukin-7 (IL-7) is required for lymphocytic growth and its survival58. Rich and Leder reported that the level of T cells increases as the IL-7 level increases59, 60.
COVID-19 is recognized as a consecutive lymphocyte destroyer since substantial long-term lymphopenia is a close universal observation in individuals with severe COVID-19, and it is linked to increased morbidity and mortality58. Putative cytokine interleukin 7 (IL-7) is required for the growth and development of lymphocytic cells61, 62. Interleukin-7 (IL-7) treatments, which have been shown to effectively reestablish lymphocyte count in a variety of viral infections, has been safely delivered in septic shock patients with lymphocyte abnormalities comparable to those seen in COVID-1963. Francois et al. saw that IL-7 can be safely administered to critically ill COVID-19 patients without inducing inflammation or pulmonary harm, and it is the marker of immunosuppression that should be severely considered when using IL-7 alone or in combination with other treatments58. In addition, the plasma expression levels of IL-2, 7, and 10, granulocyte colony-stimulating factor (GCSF), IP-10, MCP-1, macrophage inflammatory protein-1a (MIP-1A), and tumor necrosis factor (TNF-alpha) are increased in critical care patients with significant illnesses compared to non-ICU patients43.
Interleukin-8 (IL-8)
IL-8 concentration was found to be more accurate in the diagnosis of the progression of COVID-19 disease from acute to chronic than IL-7. Both mild and severe COVID-19 patients had elevated IL-8 plasma levels which increased as the disease progressed. IL-8 could therefore be cast as a biomarker for COVID-19 patients in various stages of the disease. Lili reported that the IL-8 levels in the blood were much greater in these people which makes it an excellent indicator of the COVID-19 sickness prognosis64. IL-8 is a pro-inflammatory mediator that has been implicated in tissue damage and can drive neutrophils to infected areas64. It has been documented in the topical investigation of SARS-CoV-2 infection which could enlighten the lesser manifestation of IL-8. This is crucial for chemo-attraction and neutrophil viability65. IL-8 is a proinflammatory cytokine generated by blood cells and a variety of organs, and increased concentrations of IL-8 in the blood have been linked to a variety of disorders66. The link between IL-8 and disease duration could point to a function of IL-8 signaling in COVID-19 evolution. According to new research, the onset of polymorphonuclear-myeloid-derived suppressor cells (PMN-MDSC) curtails SARS-CoV-2 specific to the T-cell responses, and the presence of PMN-MDSC at the beginning of treatment is linked to a fatal outcome in COVID-19 patients with a higher intensity of PMN-MDSC in the patients among the non-survivor group compared with the survivor group67.
Interleukin 10 (IL-10)
A type II cytokine is interleukin-10 (IL-10). The intron–exon genomic arrangement is analogous to that of other types of cytokines, and they fix to receptors with identical buildings in certain cases. The origin of the IL-10 gene in human chromosome 1q21–32 is made up of more than four axons divided by four introns68. T-helper type 2 (Th2) cells are a subgroup of regulatory white blood cell T cells called Tr1, Th1, and Th17 cells. These cells are the four key T-cell carriers of IL-1069. Other types of white blood cell such as human B cells and some granulocytes such as eosinophils and mast cells are possible sources of IL-10. Non-immune cell producers of IL-10 are epithelial cells, tumor cells, and keratinocytes70, 71, 72, 73.
In severe critically ill patients of COVID-19, the value of interleukin-10 is dramatically increased74. The tendency of SARS-CoV-2 infection to trigger IL-10 transcription in SARS-CoV. However, the significance of IL-10 as a potential immunological indicator when assessing the complexity of COVID-19 disease has been discovered75, 76. Long thought to be an anti-inflammatory or immunological inhibitory mechanism generated by a vicious cycle of proinflammatory cytokines, the presence of IL-10 in COVID-19 patients means that the serum has been suspected76, 77. Furthermore, certain researchers have advocated that recombinant IL-10 should be used as an ARDS therapy in COVID-19 patients because of its immunoregulatory and antifibrotic properties78.
In COVID-19 patients, there is the involvement of various pro-inflammatory intermediaries and vital organ demolition79. The rate of mortality could be reduced by targeting IL-10. COVID-19 individuals with severe/critical illness have drastically high serum IL-10 concentrations which correspond with disease severity74. In some critically ill individuals, IL-10 may worsen the viral sepsis-related hyper-inflammation. COVID-19 infected patients80.
IL-10 is mostly considered part of the down-regulation of the adaptive T cell response in the beginning81, 82. Xiaoling . reported that the inhibition of IL-10 signaling in COVID-19 results in extreme lung inflammation, passiveness, or constructive antiviral immunity83.
Interleukin-11
Interleukin-11 is a cytokine released by osteoblasts, fibroblasts, chondrocytes, trophoblasts, and a variety of other signaling pathways in culture84. IL-11 is easily noticeable during virally generated inflammation85. IL-11 expression can be produced by disease stimuli, implying that it can be induced by pathological stimuli as well84.
The more restricted expression pattern of the matching receptor subunits determines IL-11. IL-11R1 is produced in low levels in the central nervous system, respiratory system, thymus, spleen, cardiovascular system, bladder, kidney, muscle, small and large gut, salivary glands, bone marrow, gonads, and uterus among the two transmembrane IL-11R sub-types84. IL-11 controls blood disease and bone metabolism and prevents pro-inflammatory cytokine generation86. It's worth noting that during the early stages of SRAS-CoV infection, thrombocytopenia and lymphopenia are frequently detected in COVID-19 patients86.
Interleukin-13
Giancarlo et al. reported that IL-13 is involved in several activities such as (i) eosinophil, M2 macrophage mobilization to the lungs, (ii) the release of mucus in the air pathway and goblet cell metaplasia, (iii) enhancing the multiplication of smooth muscles, and (iv) the undergoing of fibrosis through fibroblast activation and subsequent collagen deposition87. Donlan . reported that IL-13 functions as a coordinator of pathogenic mechanisms in the lung. In COVID-19 positive individuals, the plasma altitudes of IL-13 were considerably greater than in uninfected patients88.
Patients with acute to chronic asthma have higher amounts of IL-13 in their bronchoalveolar lavage fluid, as well as a higher gene and protein expression in their bronchial mucosal tissues89. IL-13 also promotes the release of periostin, a multicellular protein involved in fibroblast stimulation and collagen gel suppleness90. To summarize, while IL-13 plays an important part in the pathobiology of asthma, there may be several redundant processes that limit the therapeutic benefits of focusing just on IL-1391.
Level of cytokine profile and COVID-19 Patient
|
Sr.No |
Cytokines |
Alteration in COVID-19 Patient |
References |
|---|---|---|---|
|
1 |
IL-2 |
↑ Patients of COVID-19 |
|
|
2 |
IL-6 |
↑ Severity level in COVID-19 |
|
|
3 |
IL-7 |
↑ Values in COVID-19 patients |
|
|
4 |
IL-8 |
↑with svereity in COVID-19 patient |
|
|
5 |
IL-10 |
COVID-19 patients have risk to an increase the value |
|
|
6 |
IL-13 |
↑ in COVID-19 |
|
|
7 |
PDGF |
↑ in COVID-19 Patient |
|
|
8 |
VEGF |
↑ in COVID-19 |
|
|
9 |
IP10 |
↑ in COVID-19 patients |
|
|
10 |
TNF-α |
↑ in COVID-19 patients |
|
Conclusion
In a viral infection, many cytokine mediators are stimulated to increase secretion and to cause a heightened severity and virulence in the body which leads to causing CRS and a high value of CRP. Many therapies are effective against CRS and harm cytokines which help to minimize the viral infection in the body. However, due to the notorious activity and physiology of virus activity, there is a need to engage in more trials and to study effective therapies.
Abbreviations
ARDS: Acute respiratory distress syndrome
CRP: C-reactive protein
CRS: Cytokine release syndrome
GCSF: Granulocyte colony-stimulating factor
PMN-MDSC: polymorphonuclear- 218 myeloid-derived suppressor cells
TNF-alpha: Tumor necrosis factor-alpha
Acknowledgments
The authors thank full to the Vice-Chancellor University of Okara. All figures were originally drawn on Biorender.com.
Author’s contributions
All authors significantly contributed to this work, read and approved the final manuscript.
Funding
None.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

