
Altered Hematological Parameters in HCV Infection: A Diagnostic Approach
- Applied Molecular Biology and Biomedicine Lab, Department of Zoology, University of Narowal, Narowal, Pakistan
- Molecular Medicine and Cancer Therapeutics Lab, Department of Zoology, Faculty of Sciences & Technology, University of Central Punjab, Lahore, Pakistan
- University of Veterinary and Animal Sciences (UVAS), Lahore, Pakistan
- Department of Zoology, University of Okara, Punjab, Pakistan
- Cell & Molecular Biology Lab, Institute of Zoology, University of the Punjab, Lahore, Pakistan
Abstract
Background: Hepatitis C is a hematogenic virus that spreads through the bloodstream. The number of cases of HCV in Pakistan, especially in the Narowal district, is increasing, and no reports regarding the changes in peripheral hematological parameters currently exist. This study aimed to show the changes in peripheral hematological parameters in hepatitis C patients due to HCV compared to healthy controls.
Methods: Blood samples from 100 controls and 100 HCV cases were collected from various hospitals in Punjab, Pakistan, between August 2021 and January 2022. The collected blood samples from healthy and HCV patients were processed for further evaluation of hematological parameters.
Results: In hepatitis C cases, the analysis also showed that there was a statistically significant difference in hemoglobin, platelets, WBCs, HCT, neutrophils and neutrophil/lymphocyte ratio NLR. On the other hand, RBCs, MCV, MCH, MCHC, lymphocytes, monocytes, and eosinophils showed no significant difference.
Conclusion: The fact that HCV patients exhibit various changes in peripheral hematological parameters may serve as a promising biomarker in HCV diagnostics and an important element in public awareness.
Introduction
The liver is involved in both homeostasis and pathology1. It is a vital organ that helps the body digest nutrients, filter blood, and fight infections. The function of the liver might be harmed when it is inflamed or damaged2. Approximately 2 million people die each year from liver disease, 1 million from cirrhosis complications and 1 million from viral hepatitis and hepatocellular cancer3. The term "hepatitis" refers to liver inflammation4. Viral, autoimmune, and drug-induced hepatitis are the most common causes of hepatitis in individuals5, 6. HCV is an enveloped RNA virus belonging to the family and the genus 7. HCV is a hepatotropic virus that causes liver damage over time, potentially leading to cirrhosis and hepatocellular cancer. Approximately 64 and 103 million persons are chronically infected worldwide8.
Acute or chronic hepatitis can result from infection by HCV. Acute hepatitis is normally asymptomatic and rarely results in liver failure. Asymptomatic acute HCV has a relatively modest clinical history, with jaundice occurring in approximately 25% of individuals. Acute infection leads to chronic infection in 60 — 80% of patients9.
Initially, HCV was considered to be transferred only via blood or its products however, recently, transmission of the virus was caused by high-risk pharmacological and sexual exposures10. In Pakistan, risk factors related to the mechanism of transmission of HCV include infectious injection (reuse of syringes or needles), barber shops, vertical transmission, ear-piercing, tattooing with unclean instruments, use of unsterilized surgical and dental devices, and intravenous drug addiction11. Chronic HCV infection is the primary cause of end-stage liver disease, hepatocellular carcinoma (HCC), and liver-related death. The natural history of chronic diseases is not fully understood. Cirrhosis develops in approximately 10 – 20 percent of patients after 20 – 30 years of HCV infection due to continuous hepatic inflammation12; the use of alcohol has been suggested as a risk factor for the advancement of liver damage in individuals with chronic hepatitis C13.
HCV prevention and control are difficult to achieve on a worldwide scale. Because there are no vaccines or postexposure prophylaxis for HCV, the main prevention efforts should focus on ensuring a safe blood supply in developing countries, promoting safe injection practices in health care and other settings, and reducing the number of persons who are beginning to inject specific drugs14.
According to data from the World Health Orginization (WHO), more than 3% of the world’s population is afflicted with HCV15. There were an estimated 56.8 million viremic HCV infections worldwide at the start of 202016. According to one study, between 2013 and 2016, approximately 2 million Americans were infected with HCV17. The total global HCV prevalence is predicted to be 2.5 percent (177.5 million HCV infected people), with viraemic rates ranging from 64.4 percent in Asia to 74.8 percent in Australia18.
In Pakistan, HCV infection is a serious medical and public health concern19, with rates ranging from 2.4 – 6.5% among adults and 0.44 – 1.6% among children20. The incidence of HCV has been observed to range from 4.1 to 36% in different parts of Khyber Pakhtunkhwa (KPK)21. The number of chronically infected people with HCV in Punjab was estimated to be 4.2 million, and in Sindh, it ranged from 7.0 to 8.0%22. In 2017, the prevalence of HCV in Pakistan's Baluchistan province was 25.77 percent23.
The following study aimed to investigate the inflammatory parameters of complete blood count (CBC) in HCV patients and to evaluate which biomarker makes a difference between healthy and normal subjects and determine the cost-effective, rapid and generally simple biomarker for the diagnosis of HCV infection among the people of Pakistan.
Methods
Subject Selection
The subjects included in this study were already diagnosed by physicians in various hospitals located in the city of Narowal from August 2019 to January 2022.
Sampling
A total of 200 blood samples were collected, out of which 100 samples were from patients diagnosed with HCV irrespective of disease stage and 100 were healthy subjects.
Complete Blood Count Test
Upon collection, CBC tests were performed on the blood samples. The results obtained were subjected to statistical analysis between the two different groups of blood samples.
Comparison of Hematology
The collected blood samples from controls and cases were processed for any significant changes in hematological parameters. Various variables or parameters were considered in the blood of control and case group samples.
Statistical means
A mean of sex was drawn among the control and case subjects, as shown in Table 1. A comparison of sexes among different groups was performed, as shown in Figures 1 and 2. Another differentiating factor was taken as the age difference between the case and control groups, as shown in Figure 3.
Mean values of the case and control groups
|
Population |
Male % |
Female % |
Total % |
|---|---|---|---|
|
HCV Case group |
47 |
53 |
100 |
|
HCV Control group |
33 |
66 |
100 |

Sex distribution of individuals in our cohort. (a) Cases contain 41% females and 59% males. (b) In the control group, there were 59% females and 41% males.
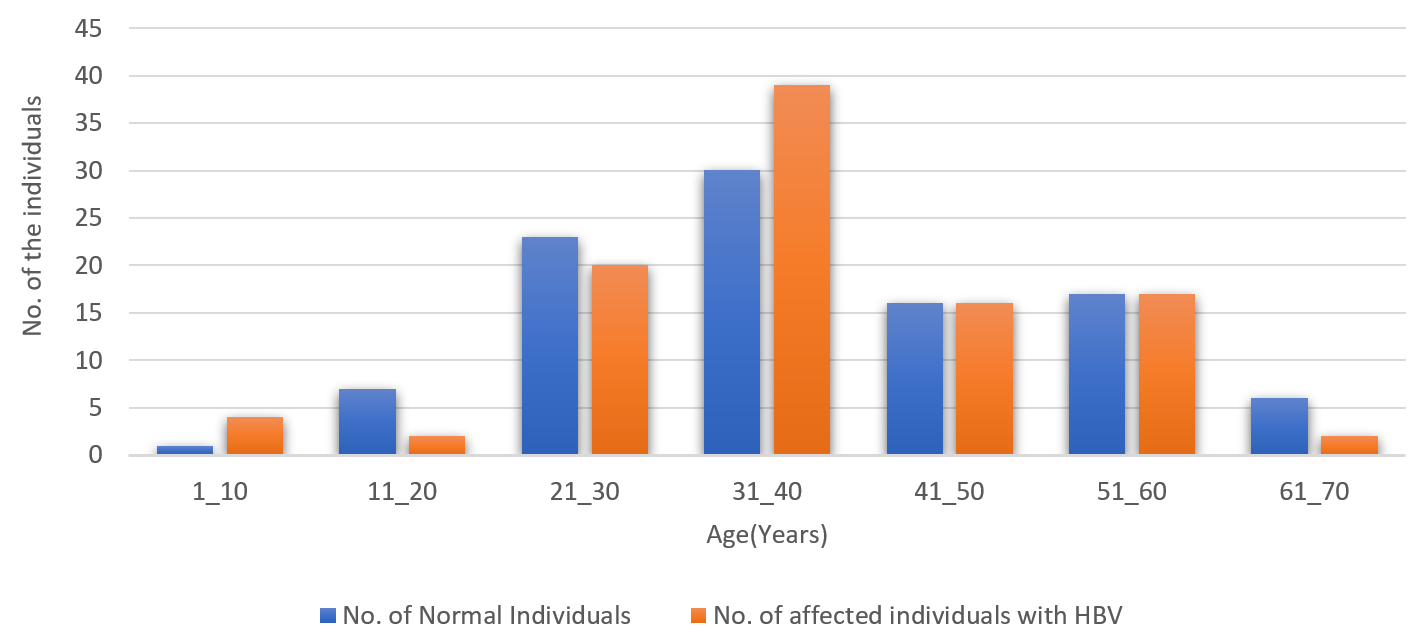
Distribution of normal and case subjects according to age. Most cases were found in the 31 – 40 and 21 – 30 age groups.

Comparison of various hematological parameters in control and case subjects. (a) HCT, (b) Hb, (c) WBCs, (h) platelets, (i) neutrophils and (m) NLR showed significant differences between the groups. However, (d) RBCs, (e) MCHC, (f) MCV, (g) MCH, (j) lymphocytes, (k) monocytes and (l) eosinophils showed no significant difference.
Results
In our cohort of HCV patients (
Discussion
The primary risk factor for HCC in Western Europe, North America, and Asia is hepatitis C virus24. Almost all HCCs linked to HCV develop in cirrhotic patients. In patients with chronic HCV infection, antiviral therapy is the only treatment to either inhibit or delay the development of HCC. Malignant behavior and histological appearance may not be strongly correlated in the early stages of HCC. In addition to preventing additional infection, the use of enhanced HCV screening techniques that can identify infection at an early stage lowers the overall number of chronic HCV patients and significantly lowers the incidence of HCC. In our observations, the levels of HB, WBCs, HCT, MCHC, platelets, lymphocytes and monocytes were significantly decreased in hepatitis C patients compared to normal individuals, and the levels of neutrophils and NLR were increased in hepatitis C patients, whereas factors such as MCV, MCH and eosinophils were not different between HCV-affected and normal individuals.
According to one investigation conducted in Taiwan, the mean PLT count in the HCV-infected group was noticeably lower than that in the control group. Given that the typical seropositive rate among blood donors in Taiwan is substantially lower than that of the general population, this discrepancy was probably caused by somewhat milder infection in the HCV-infected group in the study24. In contrast, our study also shows that the level of platelets is lower in HCV-affected individuals than in normal individuals. Our study demonstrated that the values of hematological parameters such as hemoglobin, WBCs and platelets were decreased in HCV patients compared with control subjects, and no significant differences were observed in RBCs of either group.
In another study, changes in blood composition in response to hepatitis C infection were examined. The blood composition underwent considerable modifications. RBC, WBC, HB and Plt counts were examined to evaluate changes in blood composition. This hematological condition causes a significant drop in the amount of circulating WBCs. The body's defensive system and immunity against infections are provided by leukocytes. A person may have a significantly increased chance of developing additional illnesses and infections when any of their WBC subtypes are lowered25. Similarly, our study observed a significant difference in both groups, and the WBC count was lower in HCV patients than in normal subjects. Our analysis found a significant difference in HB between both groups; the count of HB was lower in HCV patients than in healthy controls, which is due to alterations caused by the immune system in response to certain stimuli, which often lead to inflammation.
The HCV-infected group displayed noticeably greater red blood cell counts (RBC), hemoglobin (HB), and hematocrit (HCT) levels than the negative control group. The HCV-infected group also displayed noticeably greater WBC, lymphocyte, and monocyte counts (MONO) than the control group. Notably, the HCV-infected group had greater HB, HCT, and all four cell counts than the control group (RBC, WBC, lymphocytes, and MONO)24.
According to a study, end-stage renal disease (ESRD) patients who have HCV infection had greater levels of HB and HCT than those who did not have the virus. Our study showed that the levels of both parameters were decreased in hepatitis C patients26. Chronic hepatitis C may be linked to thrombocytopenia to varying degrees, according to Olariu . The platelet count falls as the disease worsens. Patients with chronic hepatitis C may experience thrombocytopenia for a variety of reasons, including bone marrow suppression, a reduction in liver thrombopoietin production, and an autoimmune mechanism. Clinical factors such as age, sex, the degree of viremia, severity of liver disease, and platelet decrease could all have an impact. Concerning their study, our investigation correlates with the decrease in platelets in hepatitis C patients compared to normal individuals27. Despite the primary results, our study has some limitations, such as the small cohort size and exclusion of different stages of hepatitis. Large cohort studies are required to account for some minor details to confirm these biomarkers.
Conclusions
The current study evaluated peripheral hematological parameters to uncover a promising diagnostic biomarker by using primary data for HCV. Large cohort studies are required for more accurate results. Finally, this study not only provides a promising approach but can also be used to create awareness among the population to prevent it and its treatment.
Abbreviations
CBC: Complete Blood Count, MCHC: Mean corpuscular hemoglobin concentration, HCT: Hematopoietic cell transplantation, HB: Hemoglobin, HCV: Hepatitis C Virus, MCH: Mean corpuscular hemoglobin, MCV: Mean corpuscular volume, NLR: Neutrophil/lymphocyte ratio, RBCs: Red blood cells, WBCs: White blood cells, WHO: World Health Organization
Acknowledgments
The authors are thankful to the Vice Chancellors of University of Narowal, Narowal, Pakistan, University of Okara, Punjab, Pakistan, and University of the Punjab, Lahore, Pakistan for providing the platform for the accomplishment of this study.
Author’s contributions
Rasheed H and Sohail A M, performed experimentation. Aman S, Afzal A and Hamid S E wrote the original draft, Shahzaman S, Abbasi M H and Sheikh N revised the final manuscript. Shah S S and Nawaz B performed statistical analysis, Tanveer S, Riasat M. edited the final manuscript. Khawar, M B supervised and proposed the idea of work. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review board approved the study, and all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

