A Case of Parabombay in Primigravida
- Department of Pathology and Transfusion Medicine, Hospital Tanah Merah, Ministry of Health Malaysia
- Department of Transfusion Medicine, Hospital Raja Perempuan Zainab II, Ministry of Health Malaysia
Abstract
Background: H antigen deficiency is an extremely rare phenotype, occurring in approximately 1 in 250,000 individuals. It is caused by the inheritance of two non-functional FUT1 alleles. The Bombay phenotype and its subtype, Parabombay, are differentiated by the expression of FUT2 (the secretory gene) in Parabombay individuals, which results in the presence of soluble H antigen in bodily secretions.
Case Presentation: We report a case of the extremely rare Parabombay A blood group detected in a primigravid woman during her first trimester of pregnancy. Early detection enabled the formulation of an appropriate birth plan, particularly regarding the procurement of H-deficient blood units. Parabombay individuals require transfusion with H-deficient blood to prevent acute hemolysis. In this case, one unit of Parabombay A blood was secured from her older brother and reserved for her delivery. The blood was irradiated prior to transfusion to inactivate residual lymphocytes and prevent transfusion-associated graft-versus-host disease (TA-GvHD), a potentially fatal complication of first-degree relative donations.
Conclusion: This case highlights the successful management of a rare Parabombay phenotype through directed donation within the patient’s family.
Introduction
The Bombay phenotype or H antigen deficiency (h/h) was first described in 1952 in Mumbai, India. It is an extremely rare phenotype, occurring in about 4 per million of the human population. It is reported in 1 per 8,000 in Taiwan, 1 per 10,000 in India, 1-2 per 300,000 in Japan, and 1 per million in Europe.Its subtype, Parabombay, is even rarer, with a reported ratio of 1:151, 2, 3, 4. Acute hemolysis can occur if ABO blood is transfused into unidentified cases due to H antigen-antibody reactions1. It is inherited in an autosomal recessive pattern5, making it possible to detect potentially similar phenotype donors, particularly among siblings.
Case Presentation
An 18-year-old lady of Malay-Thai descent, primigravida at 12 weeks of pregnancy, with an underlying Hemoglobin E trait and bronchial asthma, was referred from the primary clinic for ABO group confirmation due to blood group discrepancy.
There was an accidental finding of an ABO blood group discrepancy for forward and reverse methods during her antenatal appointment. The findings in the primary clinic and secondary hospital are summarized in
Findings in the primary clinic and secondary hospital laboratories
|
Test |
Primary clinic |
Secondary hospital |
|
Anti A |
0 |
0 |
|
Anti B |
0 |
0 |
|
Anti AB |
0 |
0 |
|
Cell A |
1+ |
2+ |
|
Cell B |
0 |
2+ |
|
Cell O |
0 |
1+ |
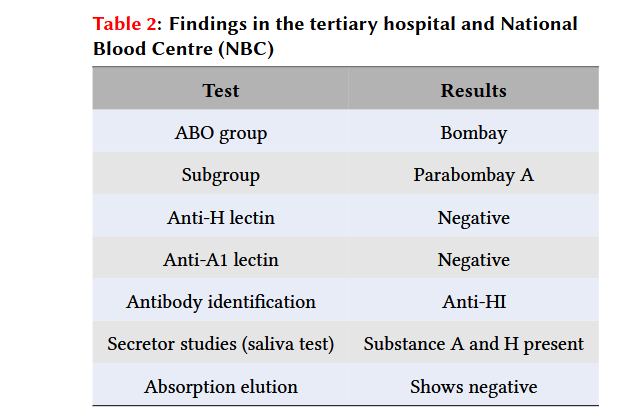
Findings in the tertiary hospital and National Blood Centre (NBC)
|
Test |
Results |
|
ABO group |
Bombay |
|
Subgroup |
Parabombay A |
|
Anti-H lectin |
Negative |
|
Anti-A1 lectin |
Negative |
|
Antibody identification |
Anti-HI |
|
Secretor studies (saliva test) |
Substance A and H present |
|
Absorption elution |
Shows negative |
A comparison between Bombay and Parabombay phenotypes
|
Characteristics |
Bombay |
Parabombay |
|
Presence of H antigen on RBC |
Not expressed |
Weakly expressed |
|
Presence of H antigen in saliva |
Absent |
Present or absent |
|
Presence of anti-H in serum |
Present |
Present |
|
Genotype |
h/h, se/se |
h/h, Se/Se or Se/se or se/se |
ABO blood grouping at the tertiary hospital was consistent with the Bombay blood group. Anti-H lectin and anti-A1 lectin were negative. Subsequently, blood and saliva specimens were sent to the National Blood Centre (NBC) for confirmation, which came back as Parabombay A with anti-HI. The saliva test showed the presence of substances A and H. The absorption elution test was negative. These findings are summed up in
She was referred to the obstetric clinic at a secondary center for follow-up and a birth plan. Hemoglobin on initial presentation was 10g/dL; she was then optimized with oral and intravenous iron throughout pregnancy.
Family screening was carried out, involving first-degree relatives, and a similar phenotype was detected in all siblings and their father. However, only her elder brother was eligible for blood donation. He successfully donated one unit of blood when the patient was at a gestational age of 36 weeks and 5 days.
The patient delivered a healthy boy at 38 weeks via spontaneous vaginal delivery the day after induction for premature rupture of membranes (PROM). Hemoglobin pre-delivery was 10.4g/dL. The delivery was complicated by a right vaginal wall hematoma that required examination under anesthesia (EUA), with a 400ml blood loss. 24 hours post-procedure, the patient had signs of tachycardia and lethargy with hemoglobin of 6g/dL, thus a single unit of red cell transfusion was commenced, followed by intravenous iron. Her overall condition improved, and she was discharged well with hemoglobin of 6.5 g/dL and a scheduled follow-up at the primary clinic to review anemia management.
Discussion
Bombay and Parabombay phenotypes are caused by the inheritance of 2 non-functional FUT1 alleles expressed on red blood cells (RBC), causing the lack of H, A, and B antigens. They are differentiated by the presence of FUT2, or the secretory gene, which is expressed in Parabombay individuals. Thus, Parabombay individuals can have a soluble form of H antigens in their body secretions, which can be absorbed into RBCs and demonstrated as weak A or B expressions. Both FUT1 and FUT2 genes are located on chromosome 191, 3. The differences between Bombay and Parabombay phenotypes are outlined in
Anti-H is a naturally occurring antibody in the plasma of people with H antigen deficiency, of the IgM class, capable of activating the complement cascade causing intravascular hemolysis. Thus, Parabombay individuals should be transfused with H-deficient red cells1.
In this case, we opted for sibling blood donation instead of frozen blood as a way to recruit new donors and also as a cost-effective measure. However, first-degree relative blood donation can lead to transfusion-associated graft-versus-host disease (TA-GvHD), which is a very rare but fatal complication following the transfusion of blood products containing viable T-lymphocytes4.
TA-GvHD occurs due to HLA haplotype sharing among family members. Thus, blood components must be irradiated to inactivate residual lymphocytes, either with gamma rays or x-rays. Red cells should be irradiated within 14 days after collection. The lifespan of irradiated RBCs is thereafter shortened to 14 days4. To tackle this shelf-life issue, we decided on a donation by 36 weeks of pregnancy and for irradiation before transfusion.
Early detection enabled a proper plan for blood support pre-delivery. We communicated with Rare Blood Donor Registry personnel at NBC regarding Parabombay A unit availability options; either a fresh or frozen unit, while simultaneously carrying out family screening that allowed us to find more individuals with the Parabombay A phenotype to be recruited as rare blood donors.
Conclusion
This case report highlights a rare case of the Parabombay phenotype in a family that was successfully transfused by directed donation.
Consent
Informed consent was not taken for this case report as it contains no image or information that can make the patient identifiable.
List of Abbreviations
NBC: National Blood Centre
PROM: Premature rupture of the membrane
EUA: Examination under anesthesia
RBC: Red blood cells
TA-GvHD: Transfusion-associated graft-versus-host disease
Conflict of Interest
All authors declare that they have no conflict of interest.
Authors’ Contribution
Nuradila Mohd Shukri: Acquisition of data and drafting the article.
Ainin Sofiya Nordin, Nurfathni Mohd Arifin, Aribah Arifin: Acquisition of data.
Nor Azah Farhah Ab Aziz, Ahmad Arif Che Ismail: Provided important intellectual content.
Rahimah Abdul Wahab: Revised the article and provided important intellectual content.
Authors’ Information
Nuradila Mohd Shukri, Ainin Sofiya Nordin, Nurfathni Mohd Arifin, Aribah Arifin: Medical officers in the Department of Pathology and Transfusion Medicine, Hospital Tanah Merah.
Nor Azah Farhah Ab Aziz: A hematopathologist based in Hospital Tanah Merah.
Ahmad Arif Che Ismail, Rahimah Abdul Wahab: Transfusion Medicine Specialists based in Hospital Raja Perempuan Zainab II.
Acknowledgment
We would like to thank the Director General of Health Malaysia for his permission to publish this article. We would also like to acknowledge the staff of Klinik Kesihatan Tanah Merah, the laboratory staff, as well as the Obstetrics & Gynecology team of Hospital Tanah Merah, the Blood Bank team of Hospital Raja Perempuan Zainab II, and National Blood Centre for their unwavering commitment to managing this case.
Funding/Support
This report received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval
Ethics approval was not required for this study.

