Monkeypox Disease: Current Knowledge, Disease potential, prevention and drug advancements
- Department of Zoology, Government College University Lahore, Punjab, Pakistan
- Department of Zoology, Government Graduate College for Women, Samanabad, Lahore, Pakistan
- Institute of Zoology, University of the Punjab, Lahore, Pakistan
- Department of Zoology, Government MAO Graduate College Lahore, Pakistan
Abstract
Monkeypox is a zoonotic viral disease with significant implications for public health. The current study aimed to review the disease, covering its etiology, epidemiology, clinical manifestations, diagnosis, outbreak potential, prevention strategies, and recent developments in drug research. Monkeypox is characterized by a spectrum of symptoms, ranging from mild febrile illness to severe systemic complications. It has significant outbreak potential, particularly in regions with limited healthcare resources and high rates of close human-animal contact. Key prevention measures, such as surveillance, public education, and vaccination, are critical for controlling transmission. Molecular diagnostic techniques, including laboratory testing, are essential for accurate case identification and outbreak monitoring. The article also examines recent advancements in drug research, including potential antiviral agents and immunomodulatory therapies. A comprehensive understanding of monkeypox is crucial for effective disease management, outbreak control, and prevention of this emerging threat.
Introduction
Each passing decade brings new viral infections that capture public attention and pose a global threat to healthcare systems. The persistent emergence and reemergence of novel zoonotic infections can be attributed to the increasing scope of globalization. Humans increasingly encroach upon natural habitats, leading to heightened interaction with infectious agents through various animal species1, 2. Among the leading zoonotic infections are cowpox, smallpox, coronavirus disease (COVID-19), and now monkeypox1.
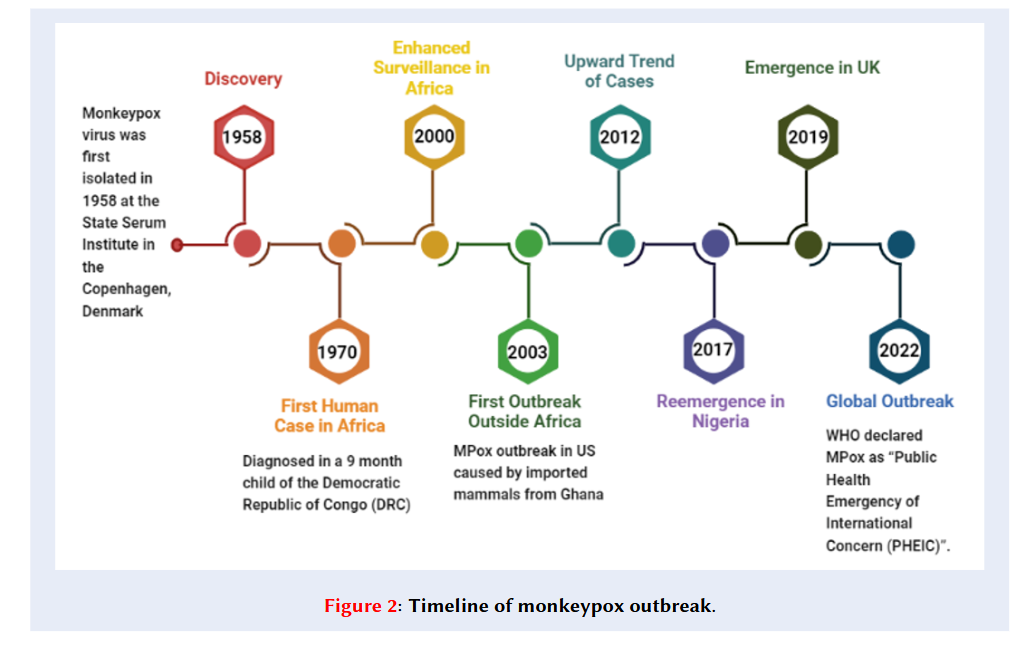
Monkeypox (MPX) is a rare neglected viral disease caused by the monkeypox virus (MPXV)13. MPX was first isolated in 1958 at the State Serum Institute in Copenhagen, Denmark, during two smallpox-like disease outbreaks in cynomolgus macaques imported to the institute4, 5. Before the eruptive stage (presence of a maculopapular rash) of the illness, no observable clinical manifestations were documented. Despite numerous animal fatalities during these outbreaks, no instances of MPX were identified in humans, implying that humans were not susceptible to the virus at that time6. However, the first MPX infection in a human was diagnosed in a 9-month-old child (suspected to have smallpox) in the Democratic Republic of Congo (DRC) in 19707. The child developed a fever followed by centrifugal skin rashes and also exhibited symptoms of otitis, mastoiditis, and tender cervical lymph nodes. MPXV was isolated from the patient's skin lesions. Although the child recovered from monkeypox, they developed measles prior to being discharged, which ultimately resulted in their demise8. Following 1970, outbreaks of MPX appeared intermittently in Central and East Africa (clade I, formerly known as the Congo Basin or Central African clade) and West Africa (clade II, formerly known as the West African clade). From September 1970 to March 1971, six more instances of monkeypox were detected in individuals residing in West African nations. After the discovery of the first case in humans, it was observed that this disease had a high prevalence in the Democratic Republic of Congo, Nigeria, Sudan, Liberia, and Sierra Leone9.
The majority of the patients were young children, and none had received smallpox vaccination (Figure 1). MPX disease has remained endemic to Africa, mainly in the DRC10. In 2003, the United States experienced its first occurrence of an MPX epidemic, consisting of 47 confirmed or probable cases. This outbreak originated from pet prairie dogs (.) that were exposed to the virus through contact with infected exotic Gambian pouched mouse imports5, 10. Subsequently, the infected prairie dogs transmitted the virus to humans, primarily affecting young adults and children11. Since 2005, numerous suspected cases have been reported annually in the DRC. The annual cases of human MPX in Africa have gradually increased since 2014. Potential reasons for the re-emergence of MPX include environmental and ecological shifts, movement of animals or humans, discontinuation of routine smallpox vaccination after its eradication in 1980, advancements in disease detection and diagnosis, and genetic alterations in the virus12.
In 2017, MPX resurfaced in Nigeria and is currently spreading among individuals throughout the nation, including travellers to other locations. In 2018, a total of five individuals infected with the virus were identified: three in the United Kingdom, one in Israel, and one in Singapore13. These imported cases were traced back to individuals who had recently travelled from Nigeria, where a significant outbreak took place during 2017–201814.
In 2022, a worldwide MPX outbreak began in the United Kingdom (UK) and spread to Europe, the United States, and other parts of the globe, subsequently reaching all six regions under the World Health Organization (WHO). Monkeypox cases were commonly observed among men who have sex with men (MSM)15. Approximately 87 thousand cases and 112 fatalities were reported across 110 countries16. This is the first time that numerous MPX cases and clusters have been reported concurrently in both non-endemic and endemic countries across widely disparate geographical areas17. On 23 July 2022, the Director-General of the World Health Organization (WHO) declared monkeypox a “Public Health Emergency of International Concern (PHEIC)” in the context of COVID-1918. MPX cases have now begun to spread across the globe (Figure 2 ). Currently, an MPX case has been confirmed in a 25-year-old Pakistani man who recently returned from Saudi Arabia. Health authorities promptly determined that this series of cases marked the beginning of a new outbreak19.

Age and Gender wise cases of MPX (Source: CDC)

Timeline of monkeypox outbreak.

Phylogenetic tree of MPXV based on NCBI taxonomic classes (Source: http://bioinfo.icb.ufmg.br/taxallnomy)

Transmission of MPXV.
Monkeypox Virus (MPXV)
The monkeypox virus (MPXV) exhibits an ovoid shape, is characterized by a double-stranded DNA structure, and has a clinical presentation similar to smallpox16, 20. MPXV belongs to the Orthopoxvirus genus in the Poxviridae family21 (Figure 3 ). The Orthopoxvirus family exhibits both antigenic and genetic similarities. The open reading frames (ORFs) within this family share more than 90% sequence identity among its various members22. The genus consists of numerous other poxviruses, such as smallpox, vaccinia, cowpox, and camelpox viruses, along with recently discovered poxviruses23. These DNA viruses with double-stranded genetic material exhibit significant genetic and antigenic similarities, leading to cross-immunity. Immunization against smallpox typically offers a certain level of defense against monkeypox24. The size of the human MPX virus is approximately 200 nm to 250 nm25. It binds to glycosaminoglycans to enter host cells. As an enveloped virus, it has been postulated to alternatively employ the classical apoptotic mimicry mechanism for entry into host cells26.
There are two genetic clades of the monkeypox virus, with their genomes differing by less than 1%, namely Clade I and Clade II. After 1970, monkeypox virus occurred sporadically in Central and East Africa (Clade I) and West Africa (Clade II)27, 28. The former virus demonstrates higher transmissibility and greater lethality, with a case-fatality ratio of 10%. Conversely, the latter virus is comparatively more self-limited, exhibiting a case-fatality ratio ranging from 3% to 6% and less human-to-human transmission29. Genomic comparative studies have revealed a 0.55–0.56% nucleotide difference between the two clades27. The recent 2022–2023 outbreak of monkeypox belongs to Clade IIb.
Transmission of MPX
There are primarily three routes of MPX transmission (Figure 4 ). First, it has a zoonotic route, ., from animal to human. This transmission involves direct contact, laceration, biting, or consumption of infected rodents. It also includes the risk of contamination for people who stay in forests or recently deforested areas, handle animal reservoirs, and are not vaccinated30. MPX infection has been observed in many species of mice, squirrels, and dormice. Therefore, the virus can also spread by touching the body fluids, blood, and mucosal or cutaneous lesions of infected animals15.
Human-to-human transmission is the second mode. It occurs through contaminated biological fluids and respiratory droplets. An infected patient’s bedding and clothes can also spread this virus. The sexual transmission of this disease is not yet clear. It has not been declared a sexually transmitted disease (STD)31. Healthcare workers, doctors, and family members handling an MPX patient are at higher risk of infection. This virus can also be transmitted to the fetus by the vertical route32.
Diagnosis
Monkeypox and smallpox have similar clinical pictures; therefore, it is necessary to make a correct diagnosis so that these diseases can be controlled33. Clinical symptoms of patients and laboratory tests are used to diagnose MPXV infection34. It is important for clinicians to study the sexual and travel history of persons who are suspected of having this infection. The same also goes for persons who are confirmed to have this infection35. Enanthemas on the mouth and tongue, as well as a vesiculopustular rash, are the hallmarks of this disease36.
Specimen analysis and MPXV determination can be performed with the help of immunological, phenotypic, and genetic methods. The key test for MPXV diagnosis is polymerase chain reaction (PCR)37. Real-time PCR is a high-quality test to detect the virus, but its availability is limited in countries with socio-economic challenges. Specimens should be taken appropriately and sent to laboratories for testing. Viral antigens can be detected by immunohistochemical methods. After 5–8 days, when a patient develops a rash, an ELISA test can be used to detect IgG and IgM antibodies against MPXV38.
Clinical manifestation and complications
Clinical manifestations of MPX are also similar to smallpox. Lymphadenopathy was observed in about 90 percent of non-vaccinated patients, and it was a distinguishing feature. It may be inguinal, submandibular, or cervical. Lymphadenopathy may be firm or tender and can be painful39. The incubation period is about 10 to 14 days. The symptoms include fever, chills, and malaise. Lymphadenopathy occurs in patients before the rash. Clinical features of this disease do not differ significantly with respect to gender or age. The rash starts to spread from the trunk region. After that, it spreads to the mouth, tongue, mucous membranes, soles of the feet, and genital areas. When the rash appears, the patient becomes contagious. At this point, it is very important to isolate the patient until the PCR result is negative. In the 2 to 4 week, lesions and pustules appear, worsening the overall condition of the patient. Due to the appearance of the rash, MPX may be misdiagnosed with other diseases. On its own, it takes 2 to 4 weeks, but it is prolonged in children under 8 years of age, immunosuppressed persons, and pregnant women. In such cases, complications arise due to eye infections, pneumonia, and encephalitis40.
Treatment, Vaccines, and Antivirals: Current Status and Future Directions
In most cases, people recover within 2 to 4 weeks. Some do not require extensive medical care. Up until now, there is no specific treatment for MPX. Therefore, treatment is usually planned according to the clinical needs of patients35. Patients are given symptomatic treatment and supportive care, and secondary infections caused by bacteria are managed41. When needed, patients are treated for skin care and hydration, and are given analgesics and antipyretic drugs.
Because of the similarity between MPX and smallpox, it has been observed that some drugs used to treat smallpox are also useful for monkeypox. Some of the important antiviral drugs are Brincidofovir, Cidofovir, Human Vaccinia Immune Globulin (VIG), and Tecovirimat42. The antiviral drug Tecovirimat was licensed by the European Medicines Agency in 202243. Tecovirimat has also been approved by the Food and Drug Administration (FDA) to treat smallpox; however, it has only been used in experimental animals against MPX and requires FDA approval for use in humans42. Initially, this drug was developed to treat smallpox. Its mode of action involves the inhibition of VP37 envelope proteins on the virus, thereby preventing viral attack and infection of host cells. Its side effects include slowed bowel movements, nausea, abdominal pain, vomiting, and headache12. Although this drug is not widely available, it is necessary to take steps to monitor clinical research data related to its use.
Cidofovir is used against cytomegalovirus retinitis in AIDS patients. It is not recommended for use in pregnant women, as embryotoxic effects were observed in animal experiments44. Brincidofovir is a prodrug of Cidofovir. Its use is more advantageous compared to Cidofovir due to lower nephrotoxicity45. Human Vaccinia Immune Globulin (VIG) is used to treat complications of the Vaccinia vaccine. This FDA-approved, purified hyperimmune globulin provides passive immunization46.
All the above discussion reveals the need to find a better drug to treat MPX with minimal side effects. Considering the importance of vaccines, it was found that two vaccines, JYNNEOSTM and ACAM 2000®, have been developed. However, these vaccines have yet to receive approval for general use by the public35.
Prevention
In the past, smallpox was eradicated from the world through a comprehensive strategy of active surveillance programs, vaccination, and public awareness47. The first step towards eradicating MPX is to stop its spread from reservoirs. Direct contact with primates and rodents should be avoided. Animal body fluids—such as urine, blood, and fecal material—should not be touched. Meat from reservoir animals should be thoroughly cooked. Pets that contract MPX should be quarantined. Patients should be handled with care. Direct contact with patients’ body fluids and respiratory droplets should also be avoided. Health care providers, including nurses and doctors, should use protective clothing, masks, and gloves48.
It is now essential to educate people about the measures to be taken to avoid MPX infection. There should be a comprehensive awareness program on SOPs, along with guidance on how to reduce the risk of exposure to the virus.
Conclusions
Monkeypox virus was mainly found in the jungles of Central and Western Africa. The rise in MPX incidence within endemic regions can be partially attributed to the waning immunity against smallpox. However, the current epidemic serves as a stark reminder that viral emergence knows no boundaries and often defies predictability in terms of its nature, target populations, and magnitude. This outbreak exemplifies how a disease originating in one region can profoundly impact non-endemic areas, presenting new clinical manifestations in different populations. To effectively combat the ongoing MPX epidemic in both endemic African regions and newly affected areas, several key priorities must be addressed. Firstly, there is a critical need to enhance awareness and education among populations, particularly high-risk groups, to prevent infection and minimize transmission. Secondly, the development of rapid and accurate point-of-care diagnostic tests is imperative for early detection, leading to improved prevention measures. Lastly, the effectiveness of existing treatments, vaccines, and vaccination strategies should be evaluated, with a focus on ensuring their availability to all affected groups and regions.
In conclusion, addressing the ongoing MPX epidemic requires a multi-faceted approach encompassing public awareness, improved diagnostics, evaluation of treatments and vaccines, equitable access, and international collaboration. By implementing these strategies, we can work towards mitigating the impact of monkeypox and preventing future outbreaks.
Abbreviations
DRC: Democratic Republic of Congo; FDA: Food and Drug Administration; MPX: Monkeypox; MPXV: Monkeypox Virus; MSM: Men having Sex with Men; PCR: Polymerase Chain Reaction; PHEIC: Public Health Emergency of International Concern; STD: Sexually Transmitted Disease; VIG: Human Vaccinia Immune Globulin; WHO: World Health Organization
Auther’s contribution
All authors contributed equally to this work. All authors read and approve this manuscript for publishing.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
None.
Funding
None.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.

