Effect of aqueous fruit extract of Xylopia aethiopica fruit on some fetal growth parameters and histology of uterus in Wistar rats
- Department of Human Anatomy, University of Maiduguri, Maiduguri, Nigeria
Abstract
Introduction: Xylopia aethiopica (X. aethiopica) fruit is widely used in Africa as a food additive. It is also employed to prevent nausea during pregnancy, aid uterine contractions during childbirth, treat menstrual flow anomalies, uterine fibroids, and malaria. With the increased consumption rate among pregnant women, especially in Nigeria, the present study aimed to evaluate the effects of the aqueous fruit extract of X. aethiopica on developing fetuses and pregnancy in rats. Methods: Twenty pregnant female rats (gestation day zero) were randomly assigned to four groups of five rats each. Group I served as the control and received distilled water, while Groups II–IV were given Xylopia aethiopica fruit extract at doses of 100 mg/kg, 200 mg/kg, and 300 mg/kg, respectively, for 18 days (days 1 to 18 of pregnancy). All rats were euthanized under ketamine injection on day 19. The abdomen was opened, and the number of live and dead fetuses and the crown-rump length were measured. The uterine horns were fixed in 10% formalin for light microscopy. Results: Administration of the aqueous fruit extract of X. aethiopica at 100 mg/kg, 200 mg/kg, and 300 mg/kg for 18 days significantly reduced (P < 0.05) the number of live fetuses, fetal viability, and crown-rump length, while increasing the number of dead fetuses compared to the control. Histological examination of the maternal uterine horn revealed distortion of the uterine epithelium in rats treated with 200 mg/kg X. aethiopica, and degenerating uterine epithelium and endometrial connective tissue in rats treated with 300 mg/kg X. aethiopica. Conclusions: Continuous consumption of X. aethiopica fruit during pregnancy could be toxic to the developing fetus and affect maternal health. Hence, caution should be exercised when consuming X. aethiopica fruit during pregnancy.
INTRODUCTION
(Dunal) A. Rich., also known as African or Ethiopian pepper, is a medicinal plant distributed in the lowland rainforests of the Guinea Savannah zones of Africa. It is cultivated in Angola, Ethiopia, Nigeria, Senegal, and Sudan1. This plant is commonly used in the preparation of African dishes, particularly in Nigeria, and in traditional medicine for managing various ailments such as skin infections, candidiasis, dyspepsia, cough, biliousness, febrile pains, bronchitis, rheumatism, dysentery, and boils2,3. Xylopia aethiopica has various pharmacological properties, including analgesic, anti-inflammatory, anti-allergic, anti-allodynic, antispasmodic, anti-hyperalgesic, antidepressant, antioxidant, and hypoglycemic effects2,4,5,6,7.
The plant has demonstrated significant progress in reproductive medicine, including hastening fetal delivery, supporting prenatal development, and serving as a pre- and post-coital contraceptive8. Herbal medicine is often preferred due to its availability and a long history of proven effectiveness9. The female reproductive system is sensitive to environmental and chemical factors such as lifestyle, radiation, drugs, and toxicants10. Exposure to these factors may lead to congenital abnormalities in fetuses and affect adult physiology11, potentially impacting reproductive capability12. Toxicity during pregnancy can exert various effects on fetal development and maternal health13. The reproductive and child health program aims to reduce maternal deaths and improve child health outcomes14. The use of plants for medicinal and traditional purposes to address health issues has been common in African and other societies for centuries15. Many herbal remedies are traditionally used as contraceptives (to prevent ovulation or fertilization), abortifacients (to prevent implantation), and as emmenagogues (to regulate uterine flow) or oxytocics (to stimulate uterine contractions, particularly to promote labor)16.
There is growing concern about health hazards associated with the consumption of food additives17,18, which may have teratogenic effects during pregnancy19. Therefore, with the increasing consumption rate of Xylopia aethiopica fruit, especially among pregnant women, studies are needed to determine the health hazards associated with its continuous exposure during pregnancy and its effects on the developing fetus.
Materials and Methods
Chemicals
Ketamine hypochlorite injection was obtained from the University of Maiduguri Clinic, while hematoxylin and eosin stains were purchased from BDH Chemical Ltd (Poole, England).
Plant Materials and Extraction
Dried fruits of were bought from Monday Market, Maiduguri, Nigeria. Aqueous extraction was carried out as described by Adienbo et al.5. The fruits were pulverized, and 330 g of the powder was soaked in three litres of distilled water. The mixture was refluxed for two hours in a continuous extraction (Soxhlet) apparatus, and the solution was filtered with a thimble to remove debris. The filtrate was concentrated to a powder using a rotary evaporator.
Animals
Forty Wistar rats (20 male and 20 female), weighing between 150–200 g, were used for this study. They were purchased from the National Veterinary Research Institute Vom, Nigeria. The rats were housed in plastic cages at the animal house of the Department of Biochemistry, University of Maiduguri, Nigeria, to acclimatize to the laboratory conditions for two weeks. They were fed with grower mash (Vital Feed, Nigeria) and water ad libitum. The rats were weighed weekly throughout the study.
Experimental Design
The fetal study was carried out according to the method described by Aouni et al.20, with minor modifications. Briefly, adult nulliparous female rats detected in the proestrus stage of the oestrous cycle were cohabited with male albino rats that had not been subjected to any experimental procedures, in a ratio of 1:1 per cage overnight. The presence of spermatozoa in a vaginal smear (identified with the aid of a light microscope) indicated successful mating and, as such, pregnancy was established. That day was considered gestation day zero. Mated females were randomly assigned into four experimental groups of five rats each. Group I served as the control and received distilled water daily, while Groups II–IV were treated with the aqueous fruit extract of X. aethiopica at doses of 100 mg/kg, 200 mg/kg, and 300 mg/kg, respectively. The administration was performed daily, starting from day 1 to 18 of pregnancy. Twenty-four hours after the last administration (day 19 of pregnancy), the rats were euthanized under ketamine injection. The abdomen was opened, and the number of live and dead fetuses, ovarian weight, as well as crown–rump length were measured. The percentage of live fetuses was estimated as the number of live fetuses divided by the total number of fetuses, multiplied by 100. The uterine horns were fixed in 10% formalin for light microscopy.
Histological study
The organs were dehydrated in graded alcohol, embedded in paraffin, and sectioned at 5µm with a rotary microtome (Leica RM2125 Rotary Microtome). The sections were stained with Hematoxylin and Eosin (H&E) and micrographs were taken using a microscope camera (AmScope, UK) at different magnifications.
Statistical analysis
Oestrous cycle and organ index data were analyzed with GraphPad Prism 7 (GraphPad, USA). One-way ANOVA and Dunnett posthoc test were conducted and the results were presented as Mean ± standard error of the mean (SEM). P < 0.05 was considered statistically significant.
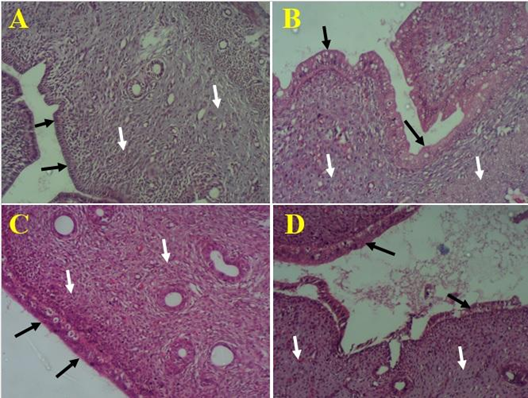
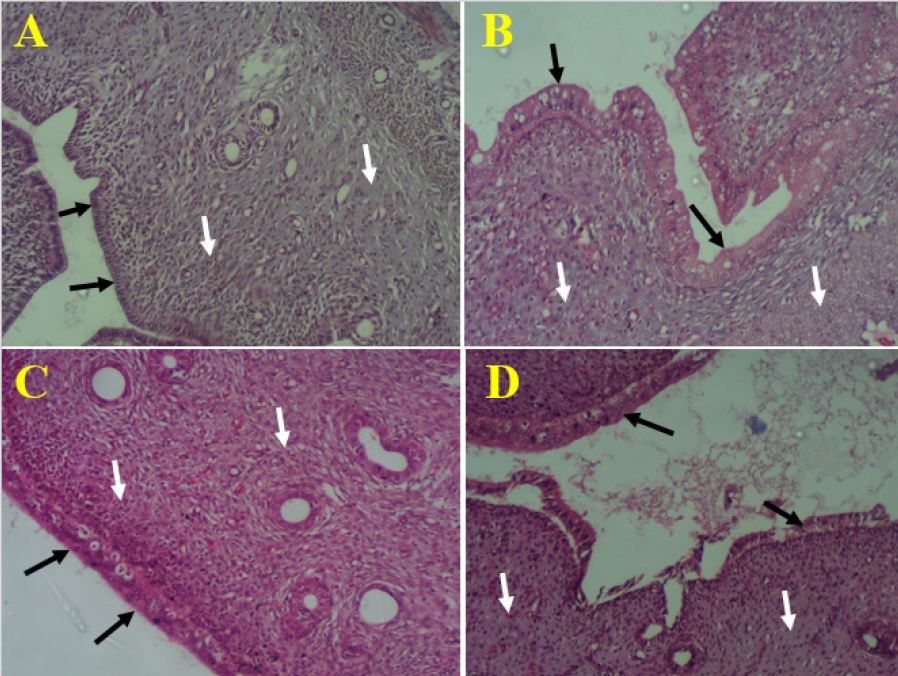
Photomicrograph of uterine horn showing endometrial epithelial lining (black arrows) and connective tissues with glands (white arrows). A= control rats, B= rats treated with
RESULTS
Maternal Body Weight
According to
Effect of Aqueous Fruit Extract of
| Groups | Dose (mg/kg) | Initial Body Weight (g) | Final Body Weight (g) | Body Weight Differences (g) | Percentage Differences % |
|---|---|---|---|---|---|
| Control | 0 | 154.48±9.44 | 217.76±14.73 | 63.28 | 40.96 |
| XA | 100 | 161.98±4.79 | 179.88±7.88 | 17.90 | 11.05 |
| XA | 200 | 150.30±4.11 | 189.44±17.63 | 39.14 | 26.04 |
| XA | 300 | 150.92±4.76 | 177.38±11.73 | 26.46 | 17.53 |
Fetal Growth Parameters
The result from the fetal study (
Effect of Aqueous Fruit Extract of
| Groups | Dose (mg/kg) | Crown Rump Length (cm) | Live Fetuses | Dead Fetuses | Fetal Viability (%) |
|---|---|---|---|---|---|
| Control | 0 | 2.90±0.05 | 6.80±0.37 | 0.00±0.00 | 100 |
| XA | 100 | 1.54±0.05* | 4.20±1.53 | 0.40±0.40* | 75 |
| XA | 200 | 1.15±0.03* | 2.80±1.70* | 0.60±0.40* | 56* |
| XA | 300 | 0.76±0.00* | 1.00±1.94* | 1.00±0.63* | 25* |
Histological Observations
The histological examination of the control rats’ uterine horn showed normal simple columnar epithelium and extensive laminar propria that bears endometrial glands and myometrium (Figure 1A). Such histoarchitecture of the uterus did not show any visible change after administration of aqueous fruit extract of at 100 mg/kg of the extract (Figure 1B). However, the uterine horn of rats that received 200 mg/kg revealed distortion of the uterine epithelium (Figure 1C) while those of rats treated with 300 mg/kg of revealed degenerating uterine epithelia and connective tissue (Figure 1D).
DISCUSSION
The present study revealed that the administration of aqueous fruit extract of X. aethiopica to pregnant rats caused an increase in maternal mean body weight. This increase may suggest that growth was not impaired by the extract in all experimental groups. Additionally, changes in maternal body weight can be used to evaluate the integrity of maternal homeostasis21. This agrees with Onyegeme-Okerenta et al.22, who reported that an aqueous extract of Millittia absences increased the body weight of pregnant rats. Although growth was not altered in all treatment groups, the extract still produced varying effects on some fetal growth parameters evaluated in this study.
Moreover, the present study showed a non-significant decrease in the weight of the maternal ovaries of rats that received the aqueous fruit extract of compared to the control. The weight of the three endocrine tissues of the ovary—the stroma, follicles, and corpus luteum—constitutes the total ovarian weight23. Hence, the reduction in ovarian weight in this study may be attributed to the absence of gonadotropin or steroid hormones due to disrupted follicular activity24.
Furthermore, this study revealed that administration of aqueous fruit extract of to pregnant rats caused significant growth retardation (decreased crown-rump length), decreased number of live fetuses and fetal viability, and an increased number of dead fetuses in the uterine horns. This could explain the histological observations in the maternal uteri of the groups that received 200 mg/kg and 300 mg/kg body weight of the extract, which revealed distention of the uterine epithelium, reduction in endometrial glands, and distortion of the uterine endometrial epithelial lining compared to the corresponding control. This may result from disruption of pregnancy by interference with mitotic division of the fetus, causing destruction of the endometrial lining of the uterus25. Likewise, the present findings are in line with reports by Onyebuagu et al.26 and Onyebuagu and Agbai27 on the activities of . They also agree with the study by Aouni et al.20, which documented that hydro-ethanolic extract of Marrubium vulgare induces severe histological changes in the pregnant rat uterus. This corresponds with the findings by Zade and Dinesh28 and Choudhary et al.29 on the mechanisms of aqueous leaf extract of Indigofera trifoliate and hydroalcoholic leaf extract of Alstonia scholaris. Additionally, reduction of endometrial glands is related to infertility and represents a defect in implantation, thus the uterus cannot accommodate a developing fetus to term30.
In addition, administration of aqueous fruit extract of caused a reduction in crown-rump length, number of live fetuses, and fetal viability, as well as an increased number of dead fetuses in experimental groups. The study has shown that nearly all drugs administered during pregnancy will, to some extent, enter fetal circulation through passive diffusion31,32. Therefore, since fruit extract was administered during the cleavage and blastula stages of rat embryonic development—before implantation—as well as at later stages of embryo and fetal development, it might interfere with the normal course of pregnancy. This could explain the negative adverse effects observed in the fetal growth parameters in the present study. This agrees with the findings of Garba et al.8, that Cissampelos mucronata root extract negatively influences reproductive outcomes in pregnant rats. It is also in line with a study by Zade and Dinesh28, who stated that Indigofera trifoliate leaf extract (aqueous, alcohol, ethyl acetate, and chloroform) harms developing fetuses. This was equally documented by Yakubu et al.33 on the adverse effects of aqueous leaf extract of Senna alata on pregnant rat outcomes.
Furthermore, the study revealed that steroids, flavonoids, alkaloids, and phenolics found in a variety of plants have antifertility activity, thereby altering normal fetal growth, development, and pregnancy34,35. Hence, in the present study, saponins, alkaloids, steroids, and flavonoid components of the aqueous fruit extract of might have exerted their antifertility effects, leading to reduced crown-rump length, number of live fetuses, fetal viability, and increased number of dead fetuses in the experimental groups. Similarly, Nwafor and Kalio36 documented that aqueous fruit extract of has strong contractile potential on uterine smooth muscles. This strong contractile property may also contribute to the observed decrease in crown-rump length and number of live fetuses, along with an increased number of dead fetuses in the present study compared to the control. This may cause irritation and stretching of the uterine cervix, subsequently stimulating the posterior pituitary gland to increase its secretion of oxytocin37. Therefore, the presence of alkaloids, flavonoids, and saponins may, in part, contribute to the oxytocic effect of the aqueous fruit extract of 38. Since the fruits and seeds of X. aethiopica are sometimes added to meals of pregnant individuals to help ease childbirth, there could be health implications for both the mother and the developing fetus if the dosage is not minimized.
CONCLUSIONS
Findings from the present study revealed that repeated consumption of fruit during pregnancy could be toxic to both the developing fetus as well as affecting maternal health. Hence, care should be taken while consuming fruit in pregnancy.
ABBREVIATIONS
ANOVA: analysis of variance, H&E: hematoxylin and eosin, SEM: standard error of the mean, XA: .
ACKNOWLEDGMENTS
None.
AUTHOR’S CONTRIBUTIONS
All authors conceived and designed the research. KOA & NID provided study materials and conducted the research. All authors analyzed and interpreted the data. KOA & NID drafted the initial manuscript. SHG & JVZ revised the manuscript. All authors approved the final manuscript.
FUNDING
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Postgraduate Board of Studies, University of Maiduguri, and performed according to the animal research: reporting of in vivo experiments (ARRIVE) guidelines.
CONSENT FOR PUBLICATION
Not applicable.
COMPETING INTERESTS
The authors declare that they have no competing interests.

