A study on the complexities of cognitive dysfunction following transient ischemic attack (TIA) subsequent to COVID-19 mRNA vaccination among the population of the Makkah region
- Department of Neurology, International Medical Center, Cairo, Egypt
- Department of Medicine, Ibn Sina National College of Medical Studies, Jeddah, Kingdom of Saudi Arabia
- Department of Microbiology and Immunology, Ibn Sina National College for Medical Studies, Jeddah, Kingdom of Saudi Arabia
Abstract
Introduction: This study assesses self-reported cognitive dysfunction following messenger RNA (mRNA) vaccination among the urban population in Saudi Arabia, addressing a critical aspect of vaccine safety. The rapid deployment of mRNA vaccines in response to global pandemics necessitates a thorough examination of potential side effects, with cognitive dysfunction emerging as a focal point because of the intricate interplay between the immune system and neurological function.
Methods: A cross-sectional study was conducted across multiple urban areas in Saudi Arabia to capture a snapshot of cognitive experiences post-vaccination. A meticulously developed questionnaire, translated and culturally adapted, collected demographic data, evaluated vaccine-related awareness, documented psychosocial factors and vaccination history, and assessed cognitive symptoms.
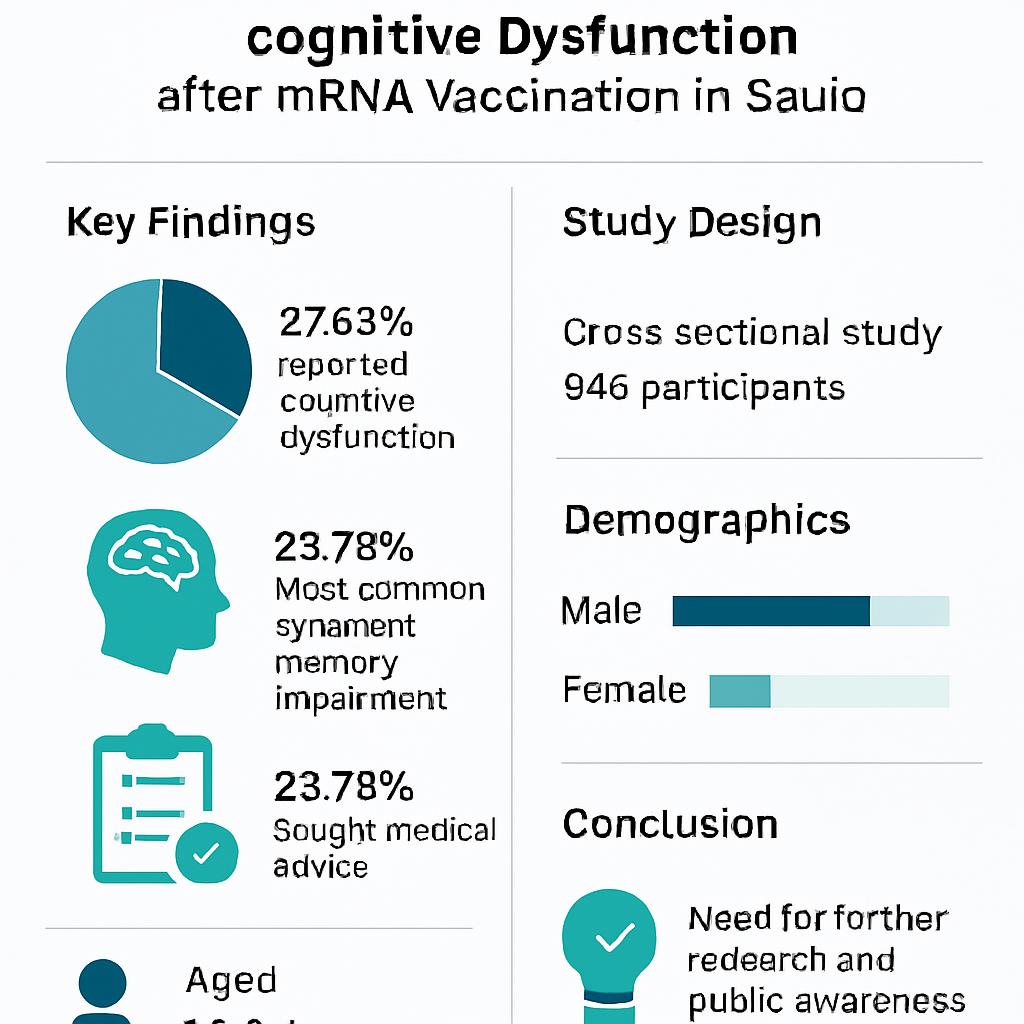
Results: Among 946 participants, 37.63 % reported cognitive dysfunction, with memory impairment being the most prevalent symptom. Approximately 23.78 % sought medical advice, indicating proactive health management, whereas 62.36 % did not, suggesting potential gaps in awareness or accessibility to healthcare.
Conclusion: The findings underscore the need for additional research, early medical evaluation, and enhanced public awareness regarding cognitive adverse events after mRNA vaccination. These data may inform clinical practice and public health policy; the study’s robust methodology and adherence to ethical standards strengthen its credibility for guiding future interventions.
Introduction
In recent years, the development and widespread deployment of messenger RNA (mRNA) vaccines, particularly those formulated to combat pandemic viral pathogens, have ushered in a transformative era in global public-health practice 1. The urgency of addressing the pandemic has spurred the rapid adoption of these vaccines, representing a monumental stride in the pursuit of effective and innovative immunization strategies. As vaccination campaigns have continued to unfold, it has become imperative to conduct thorough investigations into potential side effects and adverse reactions associated with these novel platforms. Among these considerations, the characterization of self-reported cognitive dysfunction has emerged as a crucial facet, warranting focused attention and in-depth scrutiny 2.
The human brain, as the epicenter of cognitive function, orchestrates a multitude of intricate processes that underlie perception, memory, decision-making, and overall mental acuity. Recognizing the complex interplay between the immune system and neurological function is essential, especially when introducing mRNA vaccines into the immunization landscape 3. Although the primary objective of these vaccines is to confer protection against viral infection, understanding any collateral effects on cognition remains pivotal for ensuring holistic healthcare delivery and safeguarding vaccine recipients’ well-being 4.
The present study therefore sought to investigate self-reported cognitive dysfunction among the urban population of the Kingdom of Saudi Arabia following mRNA vaccination. The urban setting provides a rich tapestry of diverse lifestyles, demographic profiles, and health-care accessibility within the Saudi context 5. By analyzing the self-reported experiences of urban residents, the study aims to elucidate the prevalence, nature, and perceived severity of cognitive dysfunction potentially associated with mRNA vaccination 6.
Urban environments, characterized by dynamic socio-economic conditions and heterogeneous health-care infrastructures, pose unique considerations for vaccine experiences 7. By centering the investigation on self-reported accounts, the study captures the nuanced and subjective features of post-vaccination cognitive phenomena 8. Moreover, focusing on Saudi Arabia—a nation with a distinctive cultural and health-care landscape—allows the findings to contribute to the broader global understanding of vaccine-related cognitive outcomes.
This work is not merely an exploration of the putative cognitive implications of mRNA vaccination; it also represents a commitment to evidence-based health-care practice and informed decision-making. The insights generated have the potential to guide clinicians in addressing cognitive concerns, facilitate the development of targeted interventions, and inform public-health strategies aimed at optimizing the vaccination experience.
By conducting this investigation within the urban enclaves of Saudi Arabia, the study aims to illuminate the connections between mRNA vaccination and cognitive function. In doing so, it intends to contribute meaningfully to the evolving discourse on vaccine safety, efficacy, and the holistic well-being of populations confronting the challenges posed by emerging infectious diseases in the twenty-first century.
Materials and Methods
Research Design
A cross-sectional study was conducted to evaluate self-reported cognitive dysfunction among urban residents of Saudi Arabia after receipt of an mRNA COVID-19 vaccine. This design allowed the capture of data at a single time point, yielding a contemporaneous snapshot of post-vaccination cognitive sequelae.
Study Location
Data were collected in urban districts within the Northern, Eastern, Western, Southern and Central provinces, thereby incorporating the diverse lifestyles, sociodemographic profiles and levels of healthcare access characteristic of Saudi Arabian cities.
Questionnaire Development
A structured questionnaire was constructed to obtain information on demographic characteristics, vaccine-related knowledge and awareness, psychosocial variables, case frequency, vaccine hesitancy, vaccination history, self-reported cognitive symptoms, healthcare-seeking behaviour, relevant medical comorbidities and validated patient-reported outcome measures.
Translation and Cultural Adaptation
The instrument underwent forward–backward translation by independent bilingual experts; discrepancies were resolved by consensus to guarantee semantic, idiomatic and conceptual equivalence while ensuring cultural appropriateness.
Questionnaire Validation
Content validity was confirmed by a panel of subject-matter experts, and a pilot study evaluated clarity and comprehensibility. Feedback from pilot participants was integrated into the final version.
Participants
Adults (≥18 years) who had received at least one dose of an mRNA vaccine were eligible. Recruitment strategies sought balanced representation across age, sex and educational strata.
Sampling Technique
Province-based stratified random sampling was used; within each stratum participants were selected by simple randomisation to achieve a sample representative of the urban population.
Sample Size
The Raosoft calculator indicated a minimum sample of 946 participants to attain a 95 % confidence level with a ±5 % margin of error, based on inclusion criteria established by a consultant intensivist.
Data Collection
Data were gathered from 17 October to 10 December 2023 via an online survey (Google Forms, English). The link was disseminated through Twitter, Telegram and WhatsApp. Electronic informed consent was obtained, and confidentiality as well as voluntary participation were emphasised.
Data Analysis
Descriptive statistics summarised participant characteristics. Associations between demographic variables and cognitive dysfunction were examined with χ² tests; multivariable models were planned to adjust for potential confounders. Statistical significance was set at p < 0.05.
Ethical Considerations
The study received approval from the relevant institutional review board and complied with the Declaration of Helsinki. Participant anonymity and data confidentiality were maintained throughout.
Result
This cross-sectional survey aimed to investigate self-reported cognitive dysfunction after mRNA vaccination among the urban Makkah population, yielding data from 946 respondents. The study provided a comprehensive overview of demographic, vaccination, medical history, and cognitive symptom data. Detailed data obtained from the questionnaires were analysed in SPSS and Microsoft Excel; frequencies, percentages, and p-values (p ≤ 0.05) are presented in
Demographic Data
| Survey Questionnaires | Response rate Absolute number (total= 946) | Response rate Percentage | p-Value (P ≤ 0.05) |
|---|---|---|---|
| Province | 0.042 | ||
| Northern | 127 | 15.8% | |
| Southern | 166 | 20.65% | |
| Eastern | 174 | 21.64% | |
| Western | 210 | 26.12% | |
| Central | 127 | 15.8% | |
| Gender | 0.051 | ||
| Male | 379 | 47.14% | |
| Female | 425 | 52.86% | |
| Age group | 0.043 | ||
| 18-24 | 194 | 24.13% | |
| 25-34 | 221 | 27.49% | |
| 35-44 | 175 | 21.77% | |
| 45-54 | 167 | 20.77% | |
| 55 and above | 47 | 5.85% | |
| Nationality | 0.037 | ||
| Saudi | 543 | 67.41% | |
| Non-Saudi | 261 | 32.49% | |
| Marital Status | 0.029 | ||
| Single | 179 | 22.26% | |
| Married | 625 | 77.74% | |
| Education | 0.039 | ||
| Under Graduate | 254 | 31.59% | |
| Graduate | 371 | 46.14% | |
| Post Graduate | 79 | 9.83% | |
| Doctorate | 17 | 2.11% | |
| Others | 83 | 10.32% | |
Vaccination Information
| Survey Questionnaires | Response rate Absolute number (total=804) | Response rate Percentage | p-Value (P ≤ 0.05) |
|---|---|---|---|
| Have you received a mRNA vaccine? | 0.011 | ||
| Yes | 747 | 92.79% | |
| No | 57 | 7.21% | |
| Specify which mRNA vaccine(s) you have received? | 0.045 | ||
| Pfizer-BioNTech | 347 | 43.18% | |
| Moderna | 221 | 27.49% | |
| Both Pfizer-BioNTech and Moderna | 178 | 22.14% | |
| None | 0 | 0% | |
| Other | 58 | 7.21% | |
| How many doses of the mRNA vaccine have you received? | 0.042 | ||
| One | 317 | 39.43% | |
| Two | 297 | 36.95% | |
| Three | 120 | 14.93% | |
| Four | 70 | 8.71% | |
Socio-demographic information
The participants represented various provinces, with East has the highest frequency (255, 26.9 %), followed by North (176, 18.6 %); these comparisons are illustrated in Figure 1. The age distribution indicated a diverse population, with significant representation across different age groups. Regarding gender, the majority were men (574, 60.67 %), and the study reflected a nearly equal split in marital status, with slightly more single participants (489, 51.69 %). The study encompassed both residents (464, 49.04 %) and non-residents (482, 50.95 %) as well as individuals with diverse educational backgrounds and occupations. Relative frequencies are displayed in Figure 2.
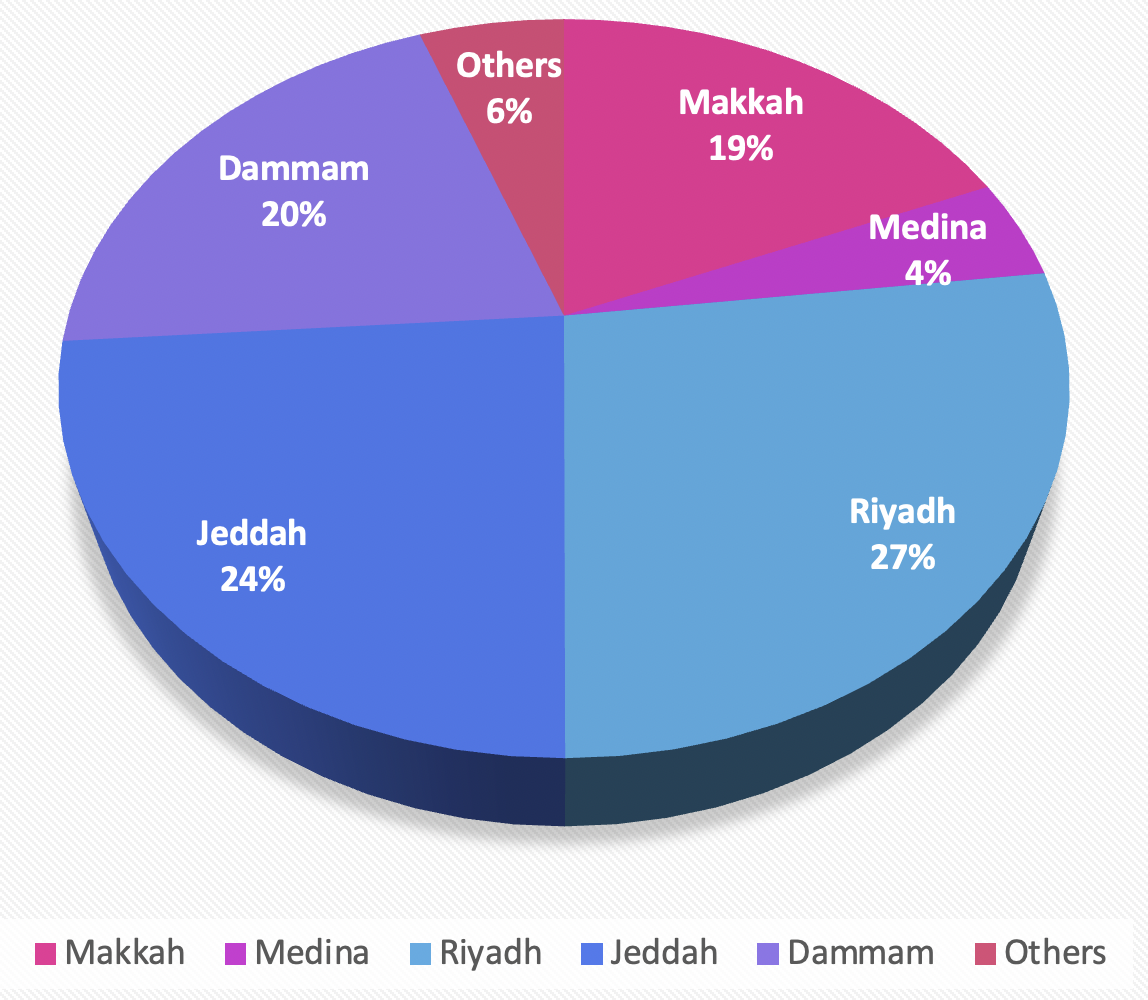
Geographic distribution of the study participants. Bar chart illustrating the frequency of participants from the primary urban centers included in the cross-sectional survey (n=946). The Eastern province contributed the largest number of respondents.
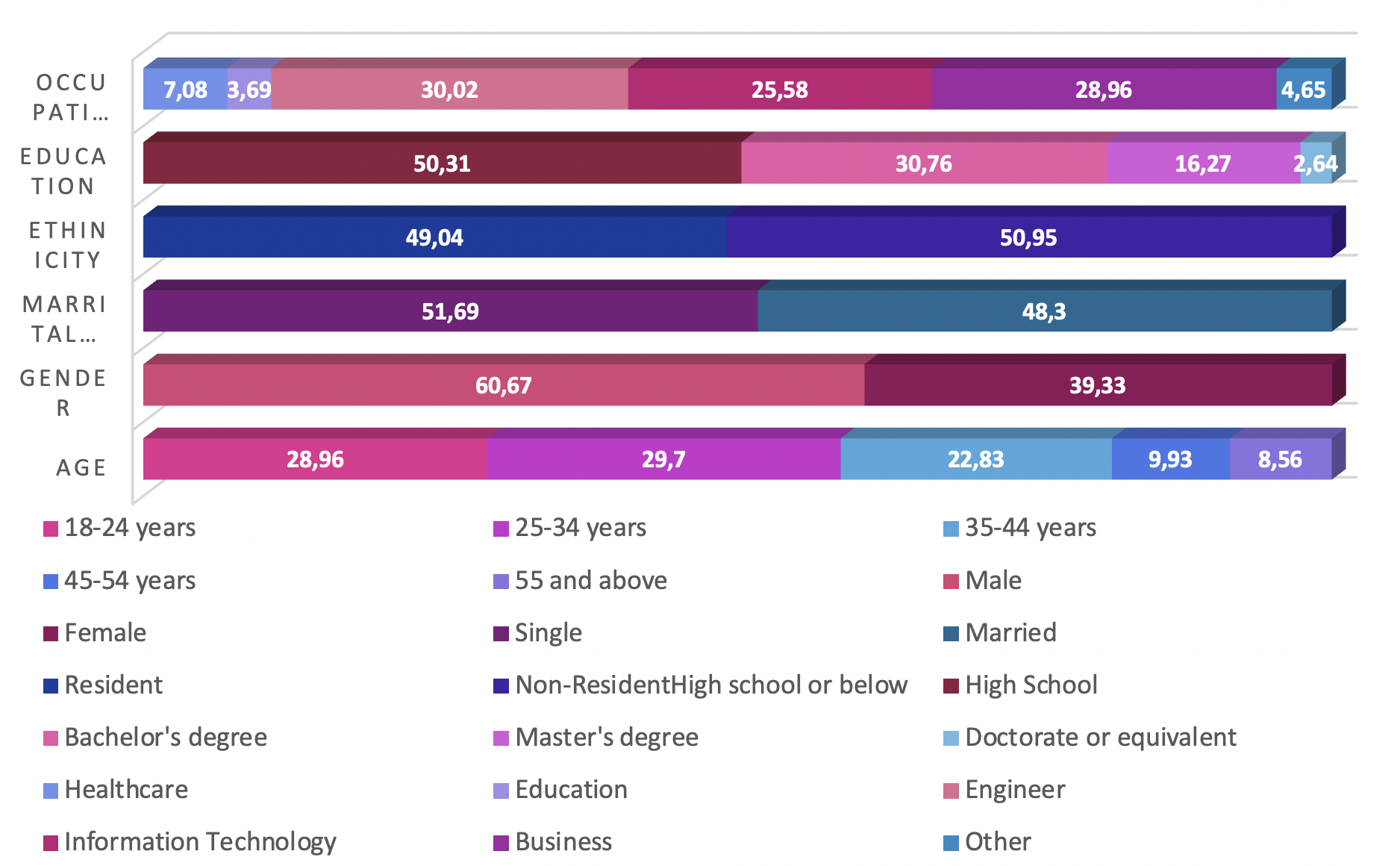
Demographic and health profile of the study cohort. Panel chart summarizing key socio-demographic variables (e.g., age, gender, marital status, residency, education, occupation) and health characteristics (e.g., Body Mass Index (BMI) classification, prevalence of chronic conditions, allergies, and smoking status) of the surveyed population (n=946).
Vaccination Information
A high proportion of participants reported having received an mRNA vaccine (874, 92.38 %), with Pfizer-BioNTech being the predominant formulation (612, 64.69 %). Most respondents had completed a two-dose regimen (354, 37.42 %). The study additionally collected comprehensive medical history data, including body-mass index, chronic conditions, allergies, and other relevant health issues. Comparative frequency distributions are presented in Figure 3.
Medical History
| Survey Questionnaires | Response rate Absolute number (total=804) | Response rate Percentage | p-Value (P ≤ 0.05) |
|---|---|---|---|
| Do you suffer from chronic diseases? | 0.049 | ||
| Yes | 387 | 48.26% | |
| No | 417 | 51.74% | |
| Do you suffer from allergies? | 0.046 | ||
| Yes | 456 | 56.72% | |
| No | 348 | 43.28% | |
| Do you have smoking related health issues? | 0.027 | ||
| Yes | 173 | 21.52% | |
| No | 631 | 78.48% | |
| Do you have Dyslipidemia related health issues? | 0.039 | ||
| Yes | 315 | 39.15% | |
| No | 489 | 60.85% | |
| Do you have Hyper tension related health issues? | 0.028 | ||
| Yes | 178 | 22.14% | |
| No | 626 | 77.86% | |
| Do you have Diabetes related health issues? | 0.027 | ||
| Yes | 221 | 27.49% | |
| No | 583 | 72.51% | |
| Do you Family history of neurological or psychiatric disorder? | |||
| Yes | |||
| No | |||
| Do you have a history of neurological or psychiatric disorders? | |||
| Yes | |||
| No | |||
| Do you take any medications that may affect cognitive function? | |||
| Yes | |||
| No | |||
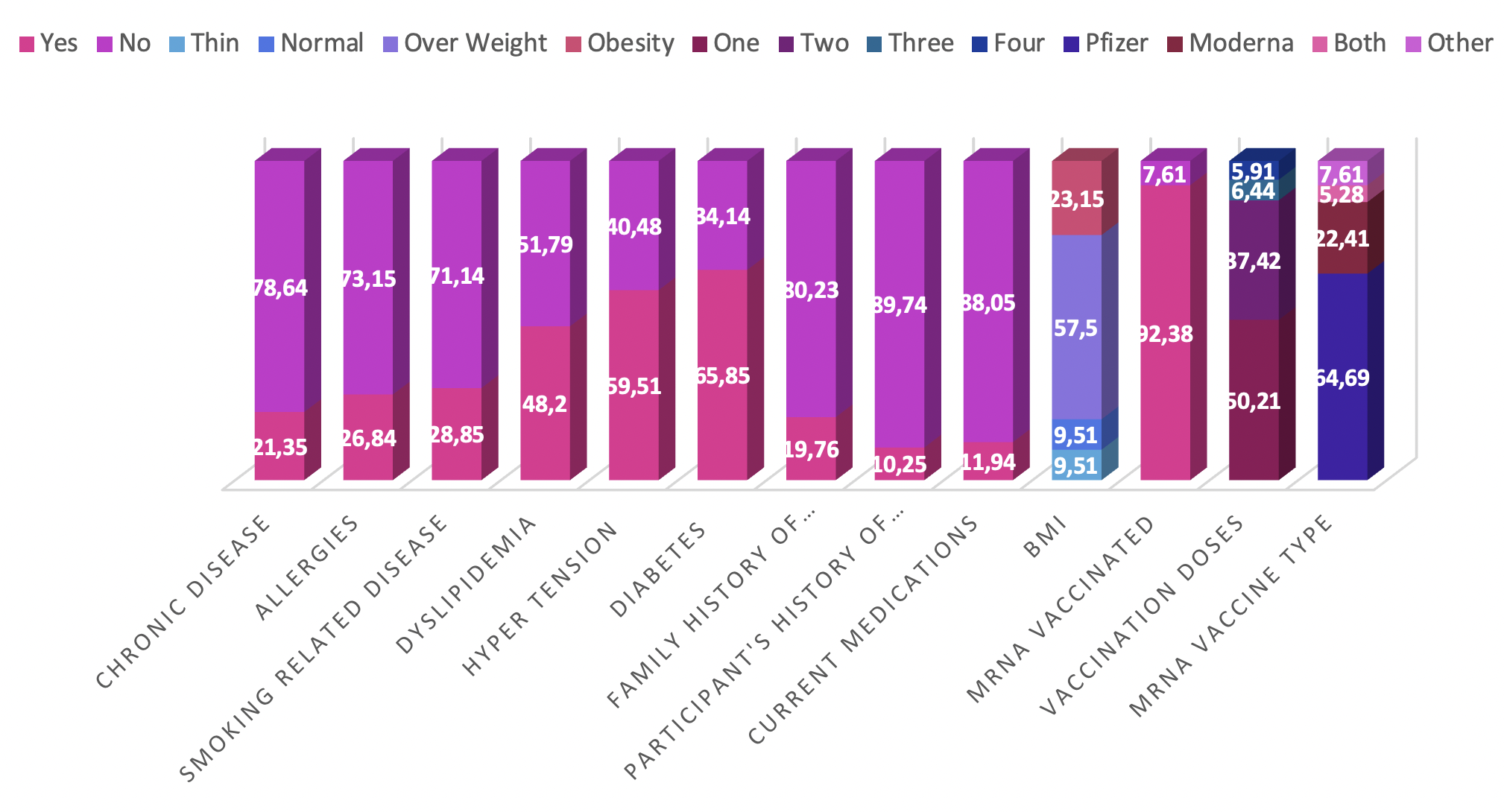
Vaccination history and medical comorbidities. Comparative charts detailing the vaccination profile of participants, including the type of mRNA vaccine received (Pfizer-BioNTech, Moderna) and the number of doses completed. The figure also illustrates the frequency of relevant medical comorbidities reported in the cohort, such as dyslipidemia, hypertension, and diabetes mellitus.
Medical History
The medical histories of the surveyed cohort reveal multiple health-related characteristics. Body mass index (BMI) classification shows that most participants are overweight (57.5%), and an additional 23.15 % are obese. Chronic diseases are present in 21.35 % of respondents, whereas allergies are reported by 26.84 %. Smoking-related disorders affect 28.85 % of the sample, and almost half (48.2 %) exhibit dyslipidaemia. Hypertension and diabetes mellitus are diagnosed in 59.51 % and 65.85 % of participants, respectively. A family history of neurological or psychiatric disorders is present in 19.76 %, and a personal history in 10.25 %. In addition, 11.94 % are currently receiving medications with the potential to impair cognitive function. Collectively, these findings delineate the health profile of the cohort and highlight conditions that may confound subsequent analyses. Comparative percentage distributions derived from the survey questionnaires are illustrated in Figure 2.
Self-Reported Cognitive Dysfunction
A notable proportion of participants reported experiencing cognitive dysfunction after mRNA vaccination (356 [37.63 %]). Memory problems were reported at varying levels of severity, with mild manifestations (394 [41.64 %]) being the most common. Difficulties with concentration, brain fog, slowed thinking, and decision-making were also described, illustrating the diverse impact on cognitive functioning. Overall, 225 participants (23.78 %) discussed these symptoms with a physician.
Among the 946 respondents, 356 individuals (37.63 %) reported cognitive dysfunction following vaccination, whereas 590 (62.36 %) did not. Of those who were symptomatic, 225 (63.20 %) consulted a physician, 131 (36.80 %) did not, and 590 participants found the question not applicable.
Severity gradations were further delineated: 112 respondents (11.83 %) reported moderate and 85 (8.98 %) severe memory impairment. Comparable distributions were observed for impaired concentration, brain fog/confusion, reduced processing speed, and indecisiveness. The perceived impact extended to work or professional duties (179 [18.92 %]), interpersonal relationships (241 [25.47 %]), academic activities (223 [23.57 %]), and daily chores or self-care (259 [27.37 %]).
Although 590 participants (62.36 %) did not seek medical advice, the remainder received guidance that included monitoring/observation, pharmacological treatment, specialist referral, lifestyle modification, or other individualized recommendations. These findings underscore the multifaceted nature of cognitive dysfunction following mRNA vaccination and highlight the importance of individualized clinical evaluation and support. The percentage distributions derived from the survey are depicted in Figure 4.
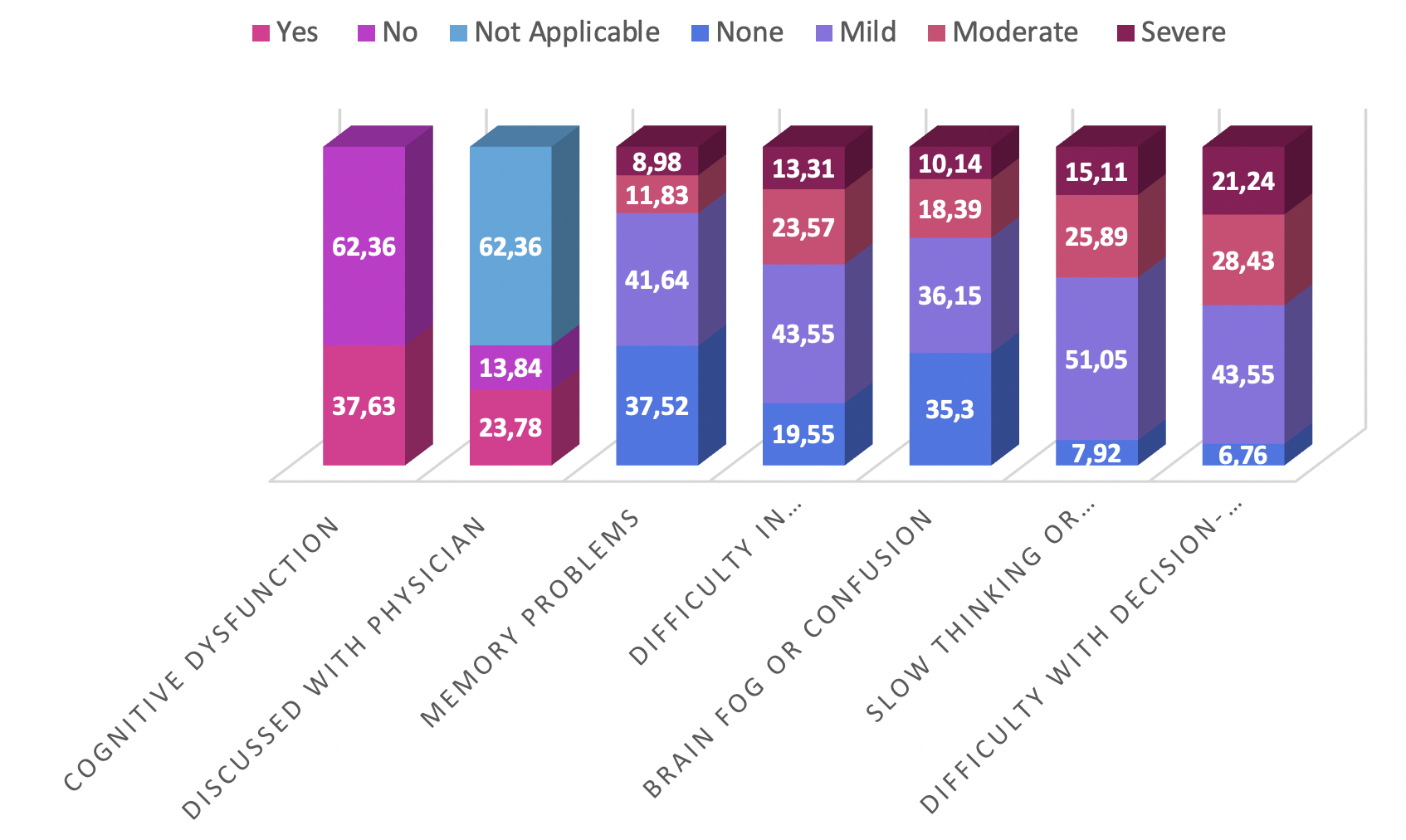
Prevalence and characteristics of self-reported cognitive dysfunction. Stacked bar and pie charts depicting the proportion of participants reporting cognitive symptoms post-vaccination (37.63%), the distribution of specific symptoms (memory impairment, concentration difficulties, brain fog), their perceived severity, and the subsequent healthcare-seeking behavior (percentage who consulted a physician).
Self-Reported Cognitive Dysfunction
| Survey Questionnaires | Response rate Absolute number (total=804) | Response rate Percentage | p-Value (P ≤ 0.05) |
|---|---|---|---|
| Have you been diagnosed for cardiac complications by a physician? | 0.024 | ||
| Yes | 218 | 27.11% | |
| No | 586 | 72.89% | |
| What is the onset duration of cardiac complications diagnosed by a physician post vaccination? | 0.032 | ||
| Less than 1 month | 117 | 14.55% | |
| 1-3 months | 53 | 6.97% | |
| 3-6 months | 31 | 3.86% | |
| 6-12 months | 11 | 1.37% | |
| More than 12 months | 6 | 0.75% | |
| Not applicable | 368 | 45.77% | |
| Did you got admitted to hospital? | 0.023 | ||
| Yes, | 218 | 27.11% | |
| No | 586 | 72.89% | |
| Have you been admitted in Critical care unit or Ward? | 0.025 | ||
| Critical Care Unit | 127 | 15.80% | |
| Ward | 91 | 11.44% | |
| No | 586 | 72.89% | |
| What is the duration of Hospitalization? | 0.024 | ||
| Less than 1 day | 56 | 6.97% | |
| 1-3 days | 89 | 11.07% | |
| 4-7 days | 67 | 8.33% | |
| 1-2 weeks | 4 | 0.50% | |
| 2-4 weeks | 2 | 0.25% | |
| More than 4 weeks | 0 | 0% | |
| Not applicable | 586 | 72.89% | |
| What is the duration for treatment? | 0.023 | ||
| Less than 1 month | 7 | 0.87% | |
| 1-3 months | 13 | 1.62% | |
| 3-6 months | 11 | 1.37% | |
| 6-12 months | 53 | 6.59% | |
| More than 12 months | 76 | 9.45% | |
| Continuous | 57 | 7.11% | |
| No current treatment | 1 | 0.12% | |
| Not applicable | 586 | 72.89% | |
| What is the treatment method? | 0.022 | ||
| Medical | 197 | 24.50% | |
| Procedure | 21 | 2.61% | |
| Not applicable | 586 | 72.89% | |
Perceived Severity and Impact
Participants were asked to rate the severity of their cognitive dysfunction on a 10-point Likert scale (1 = minimal; 10 = maximal). Responses were broadly distributed, although most ratings clustered within the mid-range (4–6). Specifically, 314 participants (33.19 %) selected a score of 5, reflecting a moderate degree of impairment.
The present study elucidates the potential association between messenger-RNA (mRNA) vaccination and self-reported cognitive dysfunction among an urban Saudi Arabian cohort. It emphasizes the need for additional research, medical surveillance, and public education regarding potential adverse effects. The current findings add valuable data to the ongoing discourse on vaccine safety and the overall well-being of individuals within the context of infectious-disease control. Comparative frequency distributions derived from the survey are presented in Figure 5.
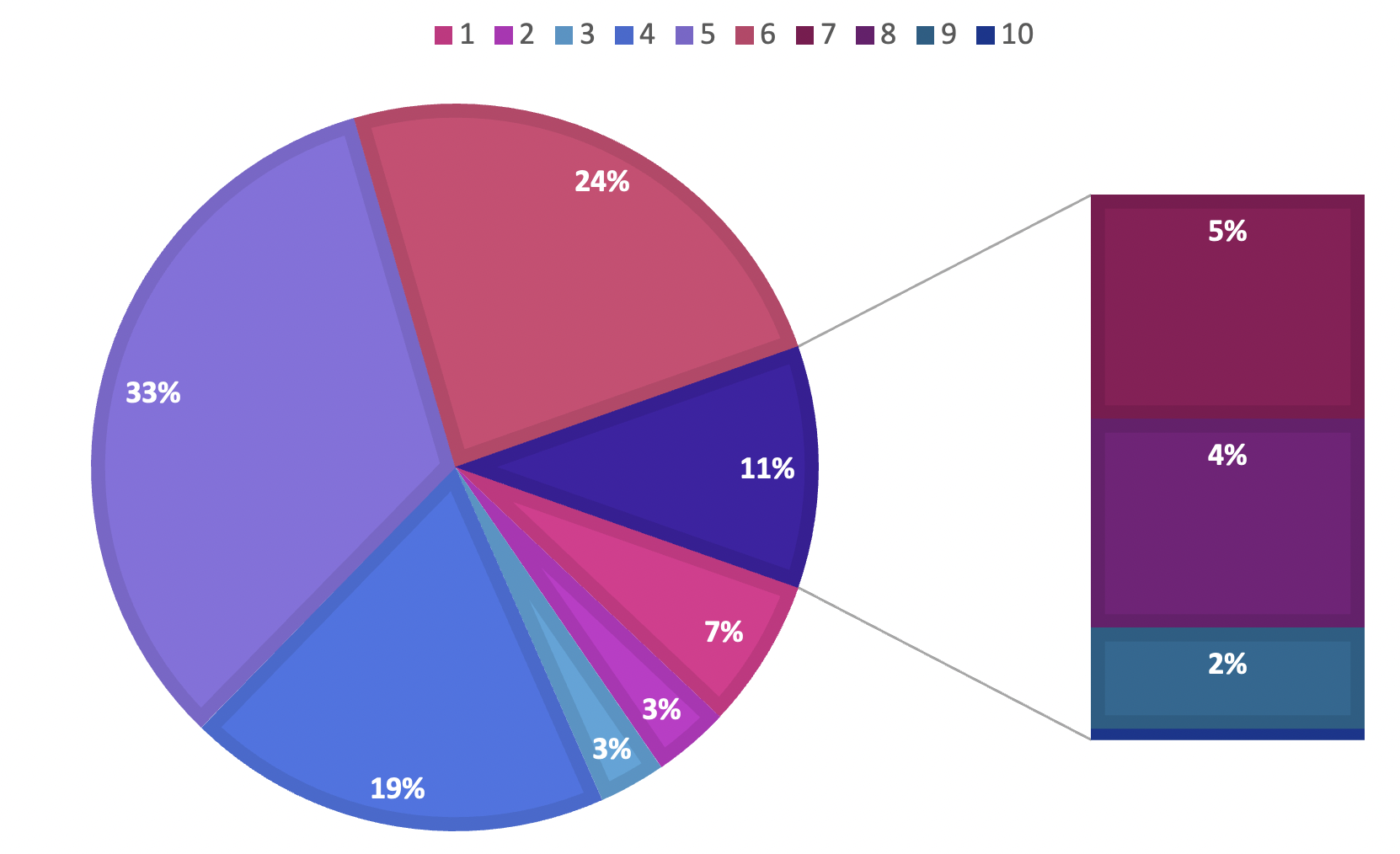
Perceived severity of cognitive dysfunction and its impact on daily life. Histogram showing the distribution of self-rated severity scores on a 10-point Likert scale, with a cluster in the moderate range (scores 4-6). A secondary chart quantifies the reported impact of cognitive symptoms on various functional domains, including occupational duties, interpersonal relationships, academic performance, and routine daily activities.
Perceived Severity and Impact
| Survey Questionnaires | Response rate Absolute number (total=804) | Response rate Percentage | p-Value (P ≤ 0.05) |
|---|---|---|---|
| How confident are you in the safety of the mRNA vaccine? | 0.044 | ||
| Very confident | 127 | 15.80% | |
| Somewhat confident | 156 | 19.40% | |
| Neutral | 384 | 47.76% | |
| Somewhat not confident | 67 | 8.33% | |
| Not confident at all | 70 | 8.71% | |
| To what extent do you believe that the cardiac complications you experienced were related to the mRNA vaccine? | 0.042 | ||
| Strongly related | 73 | 9.08% | |
| Somewhat related | 84 | 10.45% | |
| Neutral | 366 | 45.52% | |
| Not very related | 137 | 17.01% | |
| Not related at all | 144 | 17.91% | |
| What sources of information influenced your decision to receive the mRNA vaccine? | 0.040 | ||
| Healthcare professionals | 184 | 22.89% | |
| Government health agencies | 277 | 34.45% | |
| Scientific studies and research | 16 | 1.99% | |
| Family and friends | 79 | 9.85% | |
| News media | 103 | 12.81% | |
| Social media | 139 | 17.27% | |
| Other | 6 | 0.75% | |
Discussion
he findings of this study on self-reported cognitive dysfunction following mRNA vaccination among an urban population in Saudi Arabia present a valuable contribution to the ongoing discourse on vaccine safety and efficacy. To enrich our understanding, comparisons with similar studies and interpretation of the observed results provide context and identify potential avenues for further investigation.
Several international studies have explored the impact of mRNA vaccination on cognitive function, albeit with variations in methodologies and populations 9. This study aligns with emerging evidence highlighting the multifaceted nature of cognitive dysfunction following mRNA vaccination 10. The reported frequency of cognitive symptoms (37.63%) falls within the range observed in comparable studies, affirming the consistency of such experiences across diverse populations 12.
The prevalence of self-reported cognitive dysfunction in our study was somewhat higher than that reported in some studies, but remained within the expected variability 13. These discrepancies could arise from differences in sample demographics, vaccination coverage, and cultural factors influencing symptom reporting 14. It emphasizes the importance of considering regional nuances in the interpretation of cognitive outcomes after mRNA vaccination 15.
The observed heterogeneity in cognitive sequelae—including memory impairment, reduced concentration, brain fog, and impaired decision-making—highlights the complexity of individual responses to mRNA-based vaccination 16. Severity scores, reported on a 1–10 scale, demonstrated a wide distribution; nevertheless, a large subset of participants clustered in the intermediate range (scores 4–6). This pattern is consistent with the current literature, indicating that post-vaccination cognitive symptoms typically present with moderate intensity 17.
Approximately 23.78 % of respondents consulted a physician regarding their cognitive complaints 18, reflecting encouraging health-seeking behaviour and underscoring the importance of unobstructed communication between vaccine recipients and healthcare professionals 19. Conversely, 62.36 % did not seek medical advice, suggesting persisting gaps in awareness or in access to care 20.
Cognitive dysfunction adversely affected multiple life domains, including occupational performance, interpersonal relationships, academic endeavours, and routine household tasks 21. Recognition of these areas provides a more comprehensive understanding of the real-world burden of post-vaccination cognitive disturbances 22.
The present analysis therefore contributes additional data to the developing body of evidence concerning self-reported cognitive dysfunction following mRNA vaccination in an urban Saudi Arabian population 23. The patterns mirror global findings and support the call for continuous monitoring and the development of targeted interventions aimed at mitigating cognitive sequelae 24.
The investigation of self-reported cognitive dysfunction following mRNA vaccination among urban residents of Saudi Arabia provides important data on the prevalence, characteristics, and clinical consequences of post-immunization cognitive changes 25. With 37.63 % of participants reporting cognitive disturbances—predominantly memory impairment—this analysis clarifies the heterogeneous spectrum of vaccine-associated cognitive symptoms. Approximately 23.78 % of these individuals sought medical consultation, reflecting proactive health-care engagement; however, 62.36 % did not seek professional advice, indicating potential deficits in awareness or impediments to access. These findings contribute meaningfully to the global discourse on vaccine safety, underscoring the need for individualized clinical evaluation and support for those experiencing cognitive sequelae after mRNA vaccination 26. The results further emphasize the importance of public education and clear guidance for health-care providers, thereby facilitating informed decision-making, optimizing vaccination experiences, and addressing concerns related to cognitive function 27. Finally, by accounting for regional and cultural factors, this work lays the groundwork for future investigations and informs health policies tailored to the diverse requirements of different populations 28.
Several methodological strengths underscore the robustness of this cross-sectional investigation of self-reported cognitive dysfunction following mRNA vaccination among urban residents in Saudi Arabia. First, the cross-sectional design permitted data collection at a single time point, yielding a contemporaneous overview of post-vaccination cognitive experiences while optimizing resource use. The study’s broad geographic coverage across multiple urban centres in different provinces ensured a representative sample that captured heterogeneity in lifestyle, sociodemographic characteristics, and healthcare accessibility. Rigorous questionnaire development, including cultural adaptation and linguistic validation, enhanced data reliability and validity 29. Recruitment of a demographically diverse cohort with respect to age, sex, educational level, and occupation reinforced the external validity of the findings. Strict adherence to ethical standards, including institutional approval, informed consent, and data confidentiality, demonstrated a commitment to responsible research practice. Furthermore, integration of quantitative measures with qualitative reports of cognitive symptoms and healthcare-seeking behaviour provided a multidimensional perspective on cognitive sequelae after mRNA vaccination. Collectively, these attributes increase the credibility and translational value of the study, thereby advancing the evidence base on vaccine safety and neurocognitive outcomes 30.
Future investigations should explore determinants of regional variability in symptom reporting, including cultural, genetic, and health-system factors. Prospective longitudinal cohorts monitoring cognitive trajectories over extended follow-up would elucidate temporal patterns of symptom emergence and resolution. Finally, coordinated efforts among researchers, clinicians, and public-health authorities are essential to translate emerging evidence into targeted interventions for individuals who experience cognitive adverse events after mRNA vaccination.
Conclusion
This study elucidates the potential association between mRNA vaccination and self-reported cognitive dysfunction in an urban Saudi Arabian population. Its findings offer evidence to guide clinical decision-making, improve vaccination protocols, and address cognitive safety concerns; collectively, they enrich the global discourse on vaccine safety and the holistic well-being of populations confronting infectious diseases in the 21st century. The study not only constitutes an initial appraisal of such implications but also contributes to evidence-based practice, offering insights that may assist practitioners in addressing cognitive complaints and shaping public health strategies. Its rigorous methodology, heterogeneous participant representation, and strict adherence to ethical standards strengthen the validity of the findings and provide a foundation for future investigations and policies tailored to populations navigating the challenges posed by contemporary infectious diseases.
Abbreviations
BMI: Body Mass Index; COVID-19: Coronavirus Disease 2019; ICU: Intensive Care Unit; IRRB: Institutional Research Review Board; KSA: Kingdom of Saudi Arabia; mRNA: messenger RNA; SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; SPSS: Statistical Package for the Social Sciences; TIA: Transient Ischemic Attack
Acknowledgments
The authors are exceptionally grateful to the organization of Ibn Sina National College, Jeddah, KSA
Author’s contributions
All authors have made equal contribution to the work and approved it for publication
MMS: Conception and design of the work, Revisions, and final approval. ASA: Writing, Critical review of final draft, and final approval. SAA: Supervised, Reviewed, Mentored, Expertise in the study design LMH, LAF, HRA, & RMA: Conception, literature search, design, Supervision and organized the work, and final approval. AAM, SMA DMF & MSA: Investigation, conceptualization, methodology, wrote the original draft of the manuscript; TMA & TAA: conceptualization, methodology, co- wrote and organized the original draft of the manuscript. HAM, AAO & NMA: methodology, writing, reviewing, aligning and editing.
All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study, titled " A study on the post Covid-19 mRNA vaccine Transient Ischemic Attack (TIA) cognitive dysfunction intricacies amongst the population in the Makkah region" was approved by Ibn Sina National College Research Review Board Institutional Human Ethics Committee with ethical approval IRRB-03-21052023 along with the protocol identification number 71MP15052023.
Consent for publication
Not applicable.
Declaration of generative AI and AI-assisted technologies in the writing process
The authors declare that they have not used generative AI (a type of artificial intelligence technology that can produce various types of content including text, imagery, audio and synthetic data. Examples include ChatGPT, NovelAI, Jasper AI, Rytr AI, DALL-E, etc) and AI-assisted technologies in the writing process before submission.
Competing interests
The authors declare that they have no competing interests.

