Effect of an aqueous Eruca sativa extract on the restoration of hepatic function in rats with liver injury induced by the traditional Sudanese liquor (Aragi)
- Ahfad University for Women, Sudan
- Faculty of Veterinary Medicine, University of Khartoum, Sudan
- Faculty of Medical Laboratory Sciences, Shendi University, Sudan
- Ahfad University for Women
Abstract
Background: Alcoholism is a chronic , progressive disorder. This study aimed to investigate the enzymatic and histological hepatic damage induced by chronic alcohol exposure and the role of an aqueous extract of Eruca sativa in reactivating liver function.
Methods: Thirty adult Wistar albino rats were enrolled; six were sacrificed on day 0 to obtain baseline data. Blood samples were collected on days 0, 15, and 30 for biochemical analysis of hepatic enzymes. Furthermore, on day 30, six rats from each group were sacrificed for liver tissue collection. The alcohol-treated (Aragi) group was then given a calculated dose of E. sativa aqueous extract until day 60.
Results: Chronic Aragi ingestion produced a statistically significant increase (p ≤ 0.001) in serum hepatic enzymes. Administration of the extract significantly attenuated these elevations and moderately improved alcohol-induced histological changes in the liver.
Conclusion: Aqueous Eruca sativa extract may serve as a complementary therapeutic agent for alcohol-related liver injury.
Introduction
Alcoholism is a globally prevalent health disorder 1,2,3,4. It is associated with many complications, such as fatty liver, alcoholic hepatitis, and alcoholic cirrhosis 5. There are only three approved pharmacotherapies: Campral (acamprosate calcium), Naltrexone, and Antabuse (disulfiram). Natural treatments include kudzu root and St. John's wort, which have been reported to decrease alcohol intake. Eruca sativa, used as a leaf vegetable, is rich in vitamin C and potassium and is widely cultivated 6,7,8.
Excessive alcohol consumption has deleterious effects on both mental and physical health. Alcohol-induced organ damage can ultimately be fatal. Pharmacological agents currently employed for alcohol-related disorders are associated with significant adverse effects; consequently, herbal therapies have begun to replace conventional drugs 9. Phytochemical screening of grape seeds has revealed a high content of phenolic compounds, flavonoids, and terpenoids in Vitis species, whereas only a small amount of sterols and carbohydrates has been detected 10. In Sudanese traditional medicine, dates are among the most commonly used remedies for the treatment of liver diseases 11.
Some medications may exacerbate hepatic injury, as many pharmaceutical agents are intrinsically hepatotoxic, and the liver is the primary organ of detoxification. A compromised liver is unable to adequately detoxify xenobiotics, thereby perpetuating tissue injury. This observation motivated us to search for novel therapeutics that can enhance liver function without inducing additional hepatocellular damage. One such candidate is Eruca sativa. Although hepatic toxicity is prevalent worldwide, research investigating the effects of toxic compounds on the liver remains limited. The present study aimed to determine the effect of an aqueous extract of on the restoration of hepatic function in rats whose livers were compromised by Sudanese liquor ().
Methods
Study design and samples collection
This laboratory-based experimental study included 30 rats; six were sacrificed on day 0. The remaining 24 animals were randomly allocated to control and test groups, with 12 rats in each group. Rats in the test group received 500 mg/kg of an aqueous Eruca sativa extract (20 mg/mL)12. Ethical approval was obtained from the Ministry of Health Research Board, Sudan (No. AH-RES/05-021-07).
All animals were obtained from the National Centre for Research, Institute of Aromatic and Medicinal Plants (Khartoum, Almak Nimir Street). The alcoholic beverage used was Aragi, a traditional Sudanese liquor produced by fermenting dates with yeast9,12. Blood samples were collected from the retro-orbital plexus 13,14.
Preparation of Aqueous extract of aqueous extract
Four hundred and seventy-two grams of finely cut leaves of Eruca sativa were immersed in boiling water and left to stand for 24 hours. The resulting mixture was then filtered, and the filtrate was transferred to a pre-weighed flat dish. The filtrate was allowed to evaporate at room temperature, after which the dish was reweighed. The difference between the two weights represented the yield of extract in grams.
Calculation of doses
Calculation of the Aragi dose
The rats were given two cups of Aragi per day continuously for one month. (Refer to previous study which produced alcoholic liver damage after 30 days of using two cups of Aragi per day) 9,12.
The deduced formula for calculation of the rat equivalent dose was:
Calculation of the dose of Aqueous extract of
The dose of aqueous extract of used by an adult human is 500 mg/kg at the concentration 20 mg/ml.
The rat equivalent dose was calculated using the following formula:
Biochemical analysis
The levels of GOT, GPT and ALP enzymes were determined using Plasmatec kits (Manufactured by Pointe Scientific, Inc.5449 Research Drive, Canton, MI 48188.U.S.A) (9; 12), and histopathological examination was performed according to previous known methods 17,18.
Statistical methods
Data analysis was performed by SPSS software version 23.0 (IBM Corporation, Armonk, NY, USA), by using Fisher's exact test. The p<0.005 was considered as statistically significant result 19,20.
Results
Results of this study were based on observational data, biochemical analyses, and histological examinations. Approximately 30 minutes after Aragi ingestion, the participants exhibited the following behaviors: hyperactivity, unsteady gait, mutual overlapping and collisions, and changes in stool color and consistency.
Biochemical analysis
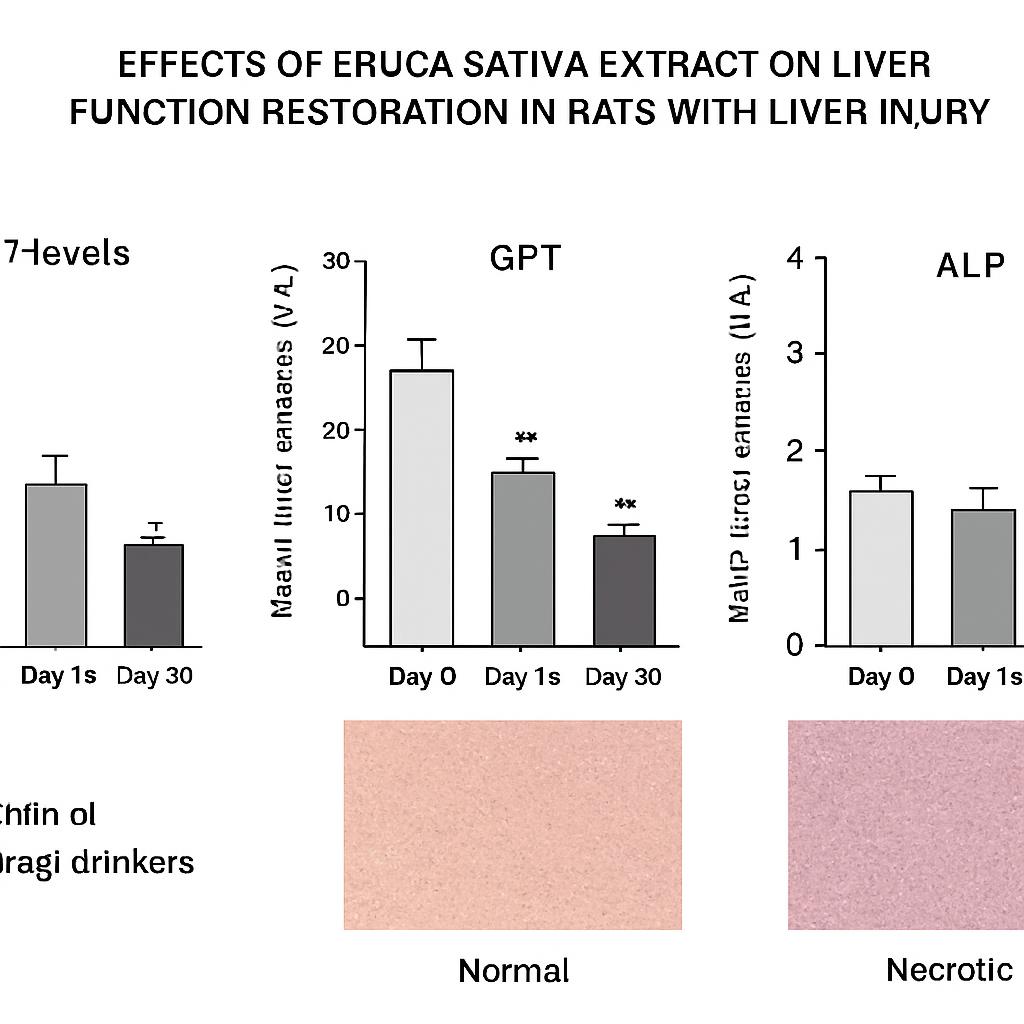
Effect of Aragi on the level of GOT
As shown in
Effect of Aragi on the level of GOT, GPT and ALP enzymes
| Time (days) | Liver enzymes results | Fisher's Exact Test P value | ||
|---|---|---|---|---|
| Control (water drinker) group | Aragi drinkers’ group | |||
| Plasma GOT (U/L) | Day (0) | 26.33 ± 1.66 | 32.42 ± 2.35 | **P≤0. 01 |
| Day (15) | 29.08 ± 2.38 | 62.50 ± 4.28** | ||
| Day (30) | 24.25 ± 2.02 | 86.67 ± 5.68** | ||
| Plasma GPT (U/L) | Day (0) | 30.42 ± 3.23 | 44.08 ± 2.18 | **P≤0. 01 |
| Day (15) | 29.50 ± 3.68 | 76.50 ± 4.14** | ||
| Day (30) | 28.25 ± 3.02 | 100.92 ± 8.13** | ||
| Plasma ALP (U/L) | Day (0) | 76.33 ± 7.91 | 81.50 ± 5.14 | **P≤0. 01 |
| Day (15) | 76.45 ± 8.58 | 172.91 ± 22.99** | ||
| Day (30) | 76.42 ± 7.27 | 268.75 ± 30.47** | ||
Effect of Aqueous extract of on normalization of the Aragi damaged GOT, GPT and ALP enzymes
The elevated GOT, GPT, and ALP activities returned to near-baseline levels (day 0) after cessation of Aragi intake and a 15-day treatment with an aqueous Eruca sativa fruit extract (Figures 1–3).
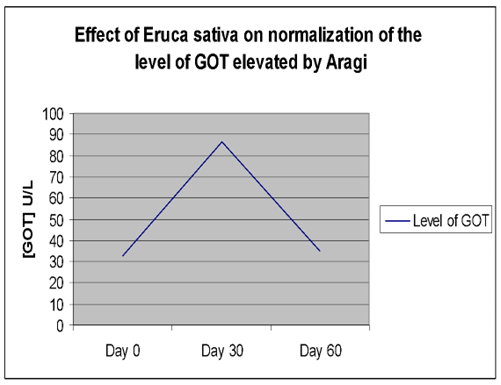
Effect of Aqueous extract of
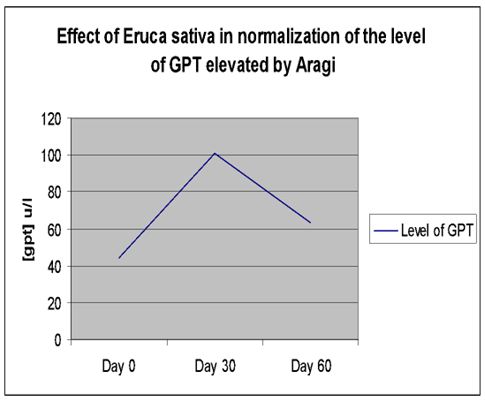
Effect of Aqueous extract of
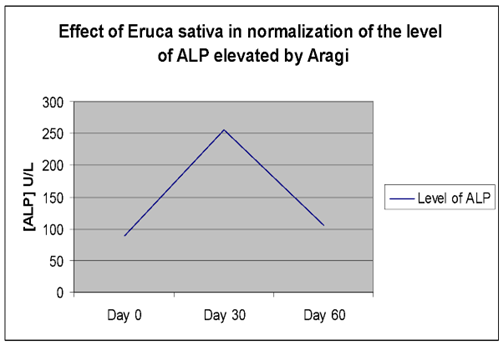
Effect of Aqueous extract of
Histological findings
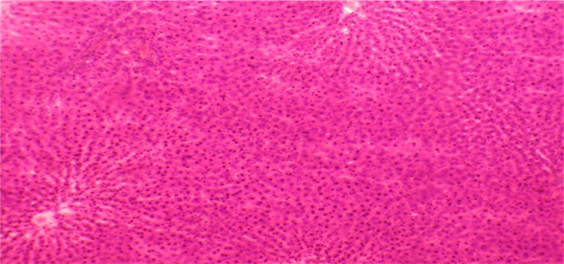
liver autopsy of control rat at day (0). Normal liver, showed no histological alteration. H&E.X 40
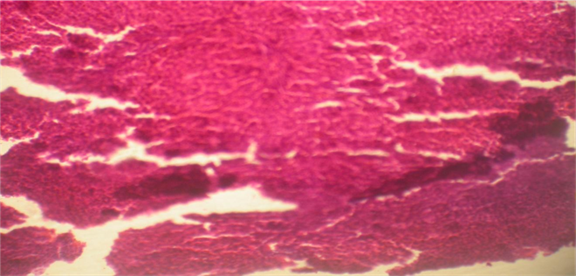
liver autopsy of Aragi drinker rat at day (30) showing severe focal necrosis, medium fibrosis, fatty degeneration and MNC infiltration. H&E.X 40.
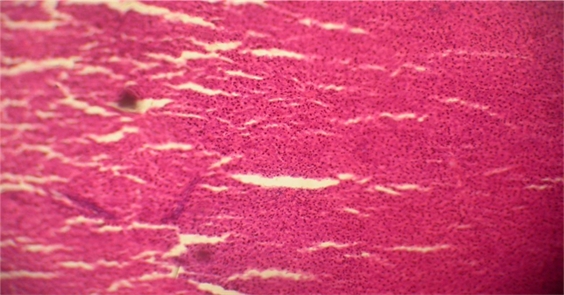
liver autopsy of camel milk drinker rat at day (60) showing medium MNC infiltration, slight fibrosis and fatty degeneration after administration of Aqueous extract of
The histological findings for the livers of the various study groups are presented in
Histological examination demonstrated normal hepatic architecture in Groups 1 and 2. Conversely, livers of Aragi-treated rats displayed mild to moderate mononuclear-cell (MNC) infiltration, mild to moderate fibrosis, and mild, moderate, or severe focal necrosis accompanied by fatty change. Supplementation with the aqueous Eruca sativa extract resulted in complete resolution of fibrosis and steatosis and moderate improvement in MNC infiltration and focal necrosis, as detailed in
Histological findings of the study groups
| In portal tract MNC infiltration | |||
|---|---|---|---|
| Histological findings | Slight (+) | Medium (++) | Severe (+++) |
| Control (Day 0)N=6 | - | - | - |
| Control (Day 30) N=6 | - | - | - |
| Aragi drinkers (day 30)N=6 | 1 | 2 | - |
| Aqueous extract of Eruca sativa (Day 60) N=3 | 2 | - | - |
| Fibrosis | |||
| Control (Day 0) N=6 | - | - | - |
| Control (Day 30) N=6 | - | - | - |
| Aragi drinkers (day 30) N=6 | 1 | 1 | - |
| Aqueous extract of Eruca sativa (Day 60) N=3 | - | - | - |
| Focal necrosis | |||
| Control (Day 0) N=6 | - | - | - |
| Control (Day 30) N=6 | - | - | - |
| Aragi drinkers (day 30) N=6 | 1 | 1 | 2 |
| Aqueous extract of Eruca sativa (Day 60) N=3 | 1 | - | - |
| Fatty degeneration | |||
| Control (Day 0) N=6 | - | - | - |
| Control (Day 30) N=6 | - | - | - |
| Aragi drinkers (day 30) N=6 | 1 | 2 | 2 |
| Aqueous extract of Eruca sativa (Day 60) N=3 | - | - | - |
Discussion
Chronic alcohol consumption induces marked hepatic injury 9,12,18,21,22,23,24,25. Consistent with our data, administration of 500 mg kg⁻¹ of aqueous Eruca sativa extract for 30 days restored biochemical and histological indices of liver injury to near-normal values, corroborating earlier studies 26,27.
Likewise, M. oleifera improved liver function tests and complete blood count parameters 28. The hepatoprotective potential of natural products has been documented extensively 29,30,31,32,33. Although conventional pharmacotherapies are efficacious, their serious adverse-effect profiles have driven interest toward botanicals such as Eruca sativa evaluated in the present work.
The study is limited by a small cohort of 30 rats distributed across several groups. This limited sample size may compromise the statistical power and external validity of the findings. Furthermore, the 60-day follow-up may be insufficient to elucidate long-term efficacy or delayed toxicities of the extract in alcohol-induced hepatopathy.
Conclusions
The present findings suggest that Eruca sativa may serve as a promising adjuvant therapy for alcohol-induced hepatotoxicity and other liver disorders. To mitigate the deleterious sequelae of alcoholism, we recommend that future investigations focus on Eruca sativa as a potential modulator of hepatic function, with particular attention to its bioactive constituents. Prolonged treatment periods should be evaluated to determine whether complete histological recovery can be achieved. Larger, long-term, randomized trials are required to substantiate these preliminary observations. Subsequent studies should incorporate a placebo- or sham-treated control group to more accurately delineate the specific effects of the aqueous extract of Eruca sativa. Dose–response analyses are also warranted to identify the optimal and safest therapeutic concentration. In addition, mechanistic research exploring the molecular pathways by which Eruca sativa exerts its hepatoprotective activity will provide a more comprehensive understanding of its therapeutic potential. Finally, future reports should expand the discussion to critically appraise the clinical implications of the data and to outline clear directions for subsequent research, thereby elucidating the significance of this intervention within the broader framework of liver-disease management.
Consent for publication
Not applicable
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not- for- profit sectors.
Authors contribution
NH and AH were involved in concepting and planning the research, HA and MA performed the data acquisition/collection, AA and NH calculated the experimental data and performed the analysis, HA and AA drafted the manuscript and designed the figures, AA and MA aided in interpreting the results. All authors took parts in giving critical revision of the manuscript.
Acknowledgement
We would like to thank the technologists of the histopathology laboratory/ Faculty of Veterinary Medicine/University of Khartoum. Great thanks to the technologists of the Department of the Quality Control / National Health Laboratory.

