
Anti-Mullerian Hormone (AMH) and Age – An Indian laboratory retrospective analysis
- Biochemistry Division, Thyrocare Technologies Limited, Plot No.D37/1,TTC Industrial area, MIDC, Turbhe, Navi Mumbai - 400703, India
Abstract
Introduction: Anti-Mullerian Hormone (AMH) is considered to be a sensitive biological indicator of the ovarian reserve among women. Produced by the granulosa cells in the ovary, AMH is also considered to be a good biochemical marker to time menopause, apart from being monitored during treatment of certain ovarian tumors. Our retrospective report is an attempt to study AMH levels across different age-groups between 18 – 50 years of age and present age-related changes in levels.
Methods: Serum AMH estimation was done in a total of 219,227 Asian Indian women using the chemiluminescent immunoassay technology.
Results: Our analysis of different age-groups with AMH levels detected a declining trend and a significant drop in levels was recorded between ages 19 – 20 years and 35 – 36 years of age at p<0.05.
Conclusion: Our report is an attempt to present age-effect on AMH levels in a pan-India cohort of Asian Indian women and analysis detected a negative correlation between age and AMH levels.
Introduction
Anti-Mullerian hormone (AMH) is secreted by the granulosa cells of the antral follicles in the ovary. It is secreted into the circulation by the small growing ovarian follicles which are </= 4 mm in diameter. Large antral follicles of size 4 – 8 mm exhibit gradual decline in AMH levels. AMH is a member of the transforming growth factor-beta family and is involved in regulation of folliculogenesis 1. It is considered to be a stable hormone as it exhibits minimal variability over a menstrual cycle and also between subsequent cycles indicating its absence in the gonadal-hypothalamic-pituitary regulatory feedback process 2,3. AMH levels have been noted to exhibit a gradual increase in levels among girls from the first day of life with the maximum levels being observed in women around 25 years of age. Among adults, its levels have been shown to gradually reduce and become undetectable among postmenopausal women as well as among women who have undergone an ovariectomy 4,5. Studies have shown AMH to play a crucial role in embryonic sex determination as the female reproductive tract has been shown to develop in its absence while it is produced by the Sertoli cells in the males. In females, the expression of AMH has been detected at day 6 after birth and is restricted to the granulose cells while among males the expression has been shown to become negligible postnatal 6.
Recommendation for AMH measurement is popular to identify premature ovarian failure, polycystic ovary syndrome (PCOS) as well as hypogonadotropic hypogonadism. Ovarian stimulation is a key aspect of assisted reproductive technology (ART) as fertility decline has been associated with increasing age and reducing ovarian reserve. Studies have indicated AMH levels to be a good pregnancy outcome predictor in ART 7. Recent studies and reports have also highlighted ovaries of Indian women to age six years faster in comparison to Caucasians and about 1 – 2% to experience signs of menopause between 29 – 34 years of age. Among women between 35 – 39 years of age, this frequency has been noted to increase up to 8% 8. The new generation AMH test has emerged as a good clinical tool to assess the fertility potential of women. Many uni- and multivariate analysis among Black and Hispanic women have identified both to exhibit 25.2% and 24.6% lower AMH levels in comparison to Caucasians 9. Very few studies have assessed the variability of AMH levels with age in a normal population as reports majorly record the relationship with reference to ART outcome10. A nomogram of serum AMH levels among healthy females is a necessity to understand the trend, factors affecting fertility as well as to generate awareness and facilitate counseling.
Our retrospective analysis is focused at studying the trend between AMH and age in an Asian Indian cohort of between 18 – 50 years of age to identify if any a cut-off age to assess significant drop in AMH levels.
Materials and Methods
Our retrospective study involving 219,227 pan-India females from age group 18 years onwards is focused on presenting relationship between age and AMH levels. The need for informed consent was deviated from as the samples were processed at a reference laboratory and serum AMH estimation was done as a part of routine screening. No identifiers apart from age and AMH levels have been considered for this analysis. Serum estimation was done using the Beckman Coulter Access® 2 Automated Immunoassay System under the principle of the chemiluminescent immunoassay (CLIA). The clinically reportable range for AMH in our CAP accredited laboratory was set at 0.3 – 10 ng/mL. Bench quality controls from Bio-Rad were used for internal quality assessment.
Results
The median AMH levels of the study cohort were assessed to be 3.0ng/mL. The cohort characteristics have been highlighted in
Study cohort summary with mean and median values of AMH across different age-groups
| Age (Years) | N | Mean AMH levels (ng/mL) | Median AMH levels (ng/mL) |
| 18 - 23 | 43349 | 6.2 +/- 4.6 | 5 |
| 24 - 26 | 43959 | 5.5 +/- 4.5 | 4.3 |
| 27 - 30 | 56447 | 4.5 +/- 4.2 | 3.3 |
| 31 - 35 | 43326 | 3.0 +/- 3.5 | 1.8 |
| 36 - 40 | 24509 | 1.5 +/- 2.4 | 0.7 |
| > 40 | 7637 | 0.6 +/- 1.5 | 0.2 |
The difference in mean AMH levels between all the age-groups was detected to be significant at p < 0.0001. This highlights a significant drop in AMH levels as an increase in age occurs. Further, a nomogram was also generated using mean and median AMH levels for age-groups 18 – 50 years of age and at an interval of 1 year. The same has been highlighted in Figure 1.
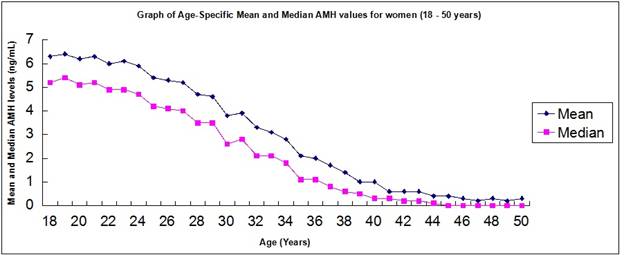
Trend correlation between mean and median levels of AMH with age 18 – 50 years.
The median levels of AMH fall below mean indicating a non-normal distribution of values. Age-group analysis between 18 – 50 years with one-year interval detected fall in AMH levels to be significant between age-groups 19 - 20 years and 35 - 36 of age at p<0.05. Further, the difference in AMH levels was significant from ages 27 – 41 years of age at p<0.0001. The graph represented in Figure 1 also highlights a negative correlation between age and AMH levels.
Discussion
A study published by Fong SL ., in 2012 generated a similar nomogram of serum AMH levels among 804 healthy females and detected a plateau until age 25 years post which an inverse correlation was observed 11. Our study detected a significant inverse correlation between age and AMH levels to begin from 27 years onwards. Another Indian laboratory survey of 29,621 samples between 20 – 55 years of age detected over 54% of women in age – group 30 – 35 years of age to harbor low AMH levels indicating fertility issues. Further, among women between 26 – 30 years of age, this study detected low AMH levels at 38% 12. Studies involving serum AMH correlation with age and ART outcome also detected a higher number of embryos when AMH levels were > 5 ng/mL and good quality embryos was higher among age-group 25 – 30 years wherein AMH levels were between 1.5 – 5 ng/mL (Pillai and Mahabalshetti (2017)). A case-control study involving 50 women subjects between age-groups 18 – 35 years, affected by PCOS and healthy detected high levels of AMH to increase the risk of PCOS and PCOS-associated hyperlipidemia and other complications 13. Technology comparison studies have also identified a decline in AMH during pregnancy to be evident when tested using Beckman Coulter in comparison to Diagnostic Systems Laboratories (AMHdsl) assay 14.
Our analysis detected a significant negative correlation between age and AMH levels from between 27 – 41 years of age and a significant age-cutoff can be noted in between 19 – 20 years of age and in between 35 – 36 years. Our analysis is one of the first to detect AMH drop among young adolescents, albeit at p<0.05 level of significance. Our study presents an effective nomogram using mean and median levels of AMH across different age-groups in a Pan-India cohort, which highlights the need for AMH testing after 27 years of age to monitor ovarian reserve.
Conclusions
AMH becomes a superior clinical tool to assess ovarian reserve as it can be measured independent of the menstrual cycle phase. Effect of ethnicity on AMH has also been recorded. The next level of clinical benefit by AMH would involve building models which could aid in predicting the period of menopause, based on which pregnancy decisions can be made.
List of abbreviations
AMH – Anti-Mullerian Hormone
ART — Assisted Reproductive Technology
CAP — College of American Pathologists
CLIA — Chemiluminescent Immunoassay
PCOS – Polycystic Ovary Syndrome
Competing Interests
The author(s) declare that they have no competing interests

