
Role of chromium supplements and diet in polycystic ovarian syndrome
- University Institute of Diet & Nutritional Sciences, Faculty of Allied Health Sciences, University of Lahore, Lahore 54000
- Memorial University of Newfoundland, Canada A1C 5S7
Abstract
Polycystic ovarian syndrome is a disorder of the female reproductive system. Obesity, hyperandrogenism, and inflammation are known to play a role in the pathophysiology of the disorder. It can cause menstrual irregularities, insulin resistance, infertility, dyslipidemia, acne, hirsutism, and glucose intolerance. A lack of a healthy lifestyle, a high intake of fatty and sugary foods, and a decrease in physical activity play a role in causing obesity and metabolic dysfunction. Chromium is an important element in carbohydrate and lipid metabolism. This review will determine how chromium improves metabolic dysfunction, lipid and carbohydrate metabolism, menstrual irregularities, and ovarian problems. We will also explore how chromium can be used as a treatment strategy for polycystic ovaries.
Introduction
PCOS is an endocrine disorder that affects females of reproductive age. Formation of cysts in the ovaries causes irregularity in the menstrual cycle. Cyst formation is mainly related to unhealthy lifestyle patterns1, and the cause of PCOS is unclear. It is a multifactorial disease with overlapping symptoms and a complex pathophysiology. It is a major cause of infertility in females2. A high level of androgens is present in PCOS females. Its clinical signs and symptoms include menstrual irregularities, acne, and hirsutism3.
The clinical manifestation of PCOS includes menstrual irregularities, acne, hirsutism, and obesity. Disturbance in the synthesis and action of androgens is typically common. It also involves impaired glucose intolerance, insulin resistance, dyslipidemia, and disturbed ovulatory function4. Some females also experience depression and anxiety, low energy levels, and fatigue due to sleep disturbances. Hormonal changes can cause abnormal hair growth. Dark skin patches are also associated with PCOS. Females suffering from PCOS gain weight easily and face difficulties in losing weight. Menstrual disturbances include oligomenorrhea, amenorrhea, and prolonged bleeding. Pelvic pain and headaches are also common with heavy periods5.
A study conducted in ISRA University in Hyderabad, Sindh concluded that there is a high prevalence of PCOS in Pakistan. The main risk factor of this disease is a genetic predisposition, and a frequently observed clinical feature is menstrual irregularities6. Women with PCOS are at a high risk of hypertension, high serum triglyceride concentration, and low serum HDL concentration7. The causes of PCOS are related to genetic and environmental factors. An unhealthy eating pattern can cause obesity which increases the level of inflammation in the body. Insulin resistance can also be a cause of PCOS. Insulin is secreted by beta cells of the pancreas and facilitates the uptake of glucose by cells. A high level of adipose tissue in the body can cause insulin resistance8.
Long-term consequences of PCOS include glucose intolerance, gestational diabetes, type 2 diabetes mellitus, hypertension, cardiovascular disease, atherogenic dyslipidemia, non-alcoholic fatty liver disease, coronary heart disease, and coagulation disorders9. Micronutrient deficiencies are also common in obese people10.
The management of PCOS includes the adoption of a healthy lifestyle pattern, a decrease in calorie intake, and increase of physical activity. Drug therapy for losing weight includes the administration of orlistat. Bariatric surgery also shows some improvement in weight loss. Weight loss in PCOS females improves hormonal, reproductive, metabolic, and cardiovascular health11. PCOS females also deal with environmental and mental stress that keeps their confidence levels low. As a consequence, they face a lot of difficulties in changing their lifestyle patterns and losing weight12.
Chromium is an essential element. It has an important role in the metabolism of glucose, insulin, and lipids. Different studies show that chromium improves hyperglycemia, insulin resistance, and fasting glucose levels13. Furthermore, chromium restriction in early life affects the insulin signaling pathway and may cause irreversible insulin resistance14. A randomized, double-blind, placebo-control study was conducted on 40 females with PCOS. The group was divided into two groups of 20 participants. One group was given 200 micrograms of chromium per day, and the other group was given a placebo for eight weeks. The result showed that chromium supplementation improves fasting glucose, insulin resistance, serum triglycerides, and increased antioxidant capacity15.
PCOS pathophysiology
Hyperandrogenism, chronic low-grade inflammation, and insulin resistance are associated with polycystic ovarian syndrome16. Elevated plasma levels of trimethylamine N oxide, an organic compound, are associated with PCOS pathogenesis without hyperandrogenism. It is also associated with high levels of systemic inflammation17.
Hyperandrogenism due to insulin can impair the beta-cell function of the pancreas, increase subcutaneous fat and lipid storing capacity, which leads to hypertrophy of intra-abdominal adipocyte and lip toxicity which increases insulin resistance18. A large number of PCOS patients with normal circulating androgen levels have high levels of follicular fluid androgens and insulin resistance19. Circadian misalignments in PCOS females characterized by a delay in melatonin offset relative to normal sleep timing can cause metabolic dysregulation. Late melatonin offset can increase serum free testosterone levels and decrease insulin sensitivity20.
Dietary treatment and supplementation
Selenium is an important element required for normal reproductive function. It involves the process of fertilization, gametogenesis, and gonadal formation21. Unfortunately, levels of serum selenium are low in polycystic ovarian syndrome females compared with healthy females22.
A study was conducted on 24 female rats to find the effect of selenium in polycystic ovarian syndrome. Rats were treated with 0.1 mg of selenium per kg of body weight. Selenium improves endocrine and metabolic phenotypes associated with PCOS comparable to metformin, and it could be used to treat PCOS23.
Zinc is a trace element, and it plays an important role in the metabolism of carbohydrates, proteins, and fats. It also takes part in the formation and release of insulin and reduces oxidative stress by synthesizing enzymes important in reducing free radicals24.
A study concluded that zinc deficiency also plays a role in polycystic ovarian syndrome pathogenesis25. A study conducted on the mouse and human skeletal muscle shows that zinc has an insulin-like effect on the cell signaling involved in glucose homeostasis. Zinc also increases glucose consumption by cells. Phosphorylation events associated with insulin signaling are also mirrored with zinc treatment26.
Vitamin E is a fat-soluble vitamin, and it consists of a mixture of tocotrienols and tocopherols. Reactive oxygenated species are one of the main causes of female reproductive disorders and damage the cell of the reproductive system. Vitamin E acts as an antioxidant and regulates the overproduction of reactive oxygenated species27. A study concluded that dietary intake of antioxidant nutrients had a protective effect on metabolic syndrome28.
Vitamin D is a fat-soluble vitamin, normally present in our body in an inactive form. It requires sunlight for activation29. Serum vitamin D level is significantly lower in PCOS females compared with healthy females. Lower vitamin status is also associated with unfavorable lipid profiles and insulin resistance30.
Vitamin D deficiency is also associated with low chances of ovulation in women with PCOS31. The vascular endothelial growth factor has been known to play a role in polycystic ovarian syndrome pathogenesis. Vitamin D supplementation decreases serum vascular endothelial growth factor and triglyceride levels in PCOS females32.

Chromium improves metabolic dysfunction.
Chromium and metabolic dysfunction
Chromium is an essential mineral for lipid and carbohydrate metabolism33. Chromium enhances insulin signaling pathways inside the cells by decreasing cholesterol content in membranes to enhance glucose transportation34. Restriction of chromium in early life affects the insulin signaling pathway and may cause irreversible insulin resistance.
A study conducted by Elizabeth Joseph showed that chromium supplementation lowers triglycerides levels in diabetic patients35. In addition, chromium malate improves lipid metabolism by reducing total cholesterol, triglycerides, and LDL levels and increasing serum HDL levels36 as shown in Figure 1.
A study concludes that chromium increases insulin receptor kinase activity37. Furthermore, a study conducted on mice chromium enriched bacillus subtilis enhanced the expression of insulin receptors and improved chromium concentration in body tissues. It also decreased the levels of plasma glucose and LDL cholesterol and increased the level of HDL cholesterol38.
In a study complex of chromium with sulfated rhamnose, polysaccharides are synthesized to assess the effect on type 2 diabetes mice. Mice were fed with a high sucrose and high-fat diet. SPRC treatment was provided to them for 11 weeks daily. SPRC treatment activates the signaling pathway of glycogen synthesis and enhances the transportation of glucose. It increases the glycogen content of tissues, reduces body mass, and improves the oral tolerance of glucose39.
In a study conducted on 24 female mice to assess the effect of chromium supplementation, the result showed that chromium supplementation improves the level of fasting glucose and fasting insulin40.
Chromium and BMI
High BMI levels mean a high percentage of fat in the body which worsens the condition of polycystic ovarian syndrome. Abnormal subcutaneous fat concentration increases the production of adipokine41. Another study concluded that central obesity increases the apolipoprotein B / apolipoprotein A1 ratio. A polyprotein includes the potentially antho-genic compounds. A polyprotein A1 is the main component of HDL42.
In a study, the use of chromium and carnitine as co-supplements decreases body weight, BMI, and fasting glucose, and improves the lipid profile which has a beneficial effect in polycystic ovaries43.
Supplementation of chromium picolinate modifies the mRNA levels related to glucose metabolism and lipogenesis to impart a positive effect on glucose homeostasis, leading to a beneficial effect in the whole-body composition and on BMI44.

Chromium improves lipid profile.
Chromium and lipid metabolism
Dyslipidemia is common in women with polycystic ovaries and involves a disturbance in lipid metabolism and lipid profile. There is a mostly abnormal level of cholesterol present45. A study concluded that a high-fat diet prior to puberty plays an essential role in the development of polycystic ovaries as it causes ovarian changes and disturbances in the metabolism46.
An experiment was conducted on broilers to assess the effect of chromium on lipid metabolism. Chromium decreases the fat percentage in the abdomen, as shown in Figure 2. It also decreases the activity of fatty acid synthase, hormone-sensitive lipase, acetyl coA carboxylase, and lipoprotein lipase. There is also an increase in fatty acid synthase and lipoprotein lipase genetic expression47.
Hepatic steatosis is a condition in which there is an accumulation of abnormal fat percentage in the liver. As a result, the metabolism of lipids is disturbed in this disease48. Chromium reduced the accumulation of fatty acids and lipids in hepatic steatosis, which shows that chromium has a beneficial effect on the metabolism of lipids49.
mRNA expression related to lipid metabolism is regulated by chromium. There is improvement in triglycerides, LDL, and cholesterol; all negative biochemical indicators related to lipid metabolism. It also regulates lipid accumulation, and it can be used in treating hyperlipidemia50.
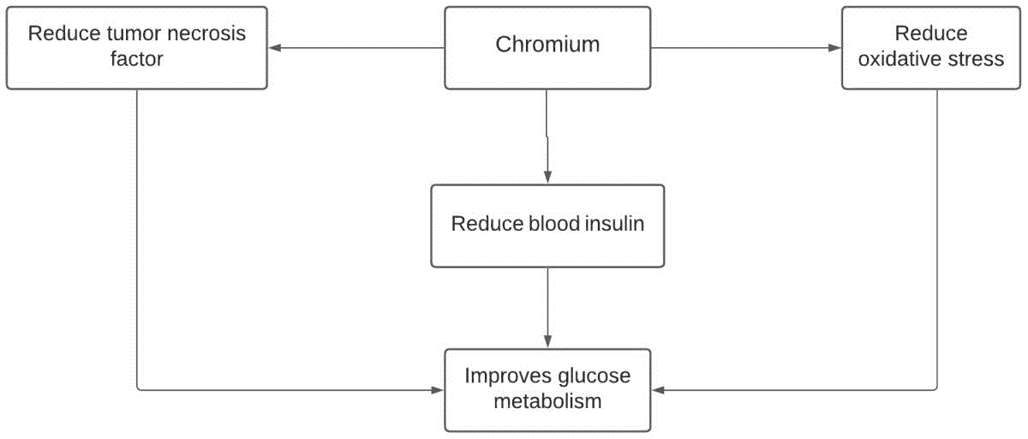
Chromium improves glucose metabolism.
Chromium and blood glucose
A study was conducted on type 2 diabetes patients to assess the effects of low and high plasma chromium levels. Hyperglycemia, hyperinsulinemia, and insulin resistance were associated with low plasma chromium levels. Favorable fat distribution was associated with high plasma chromium levels51. A study conducted on non-diabetic individuals reveals that high urinary chromium excretion is associated with high levels of insulin resistance52.
A study was conducted on streptozotocin-induced diabetic rats. They were administered chromium picolinate (1 mg per kg of body weight daily) for four weeks. The result showed improvement in plasma glucose levels as shown in Figure 3, which was beneficial for polyphagia, polydipsia, and weight loss as shown in Figure 2. It also normalizes the glycogen content in the liver. It increases the activity of glycolytic enzymes and decreases the activity of the gluconeogenic enzyme53. A study was conducted on type 2 diabetic rats treated with chromium malate for eight weeks. A high dosage of chromium malate was shown to increase the antihyperglycemic activity, and the levels of hepatic glycogen, glucokinase, and glucose 6 phosphatase dehydrogenase36.
A 90-day study was conducted on obese individuals with type 2 diabetes mellitus provided with supplementation of chromium picolinate with biotin, which improved fasting glucose levels in all participants33. In another study conducted on type 2 diabetes, patient’s supplementation of chromium and cysteine was shown to improve insulin resistance and reduce blood levels of insulin, tumor necrosis factor, and oxidative stress54.
Chromium and hormonal imbalance in the menstrual cycle
The menstrual cycle consists of the following phases: menstrual phase, follicular phase, pre-ovulatory phase, luteal phase, and pre-menstrual phase. In the first half of the menstrual cycle, estrogen levels are low and the follicle-stimulating hormone and luteinizing hormone peak. In the second half of the menstrual cycle, estrogen level rises55.
A study was conducted on 40 female patients with PCOS to find the disturbance in their hormonal profile. The samples of blood were taken during days 2 to 4 of the menstrual cycle. The result of these PCOS patients were compared with the healthy female control group. The hormones evaluated in this study included the follicle-stimulating hormone, oxytocin, prolactin, testosterone, and the luteinizing hormone. The result showed an elevated level of all these hormones in PCOS females56.
A study was conducted on 54 participants to assess the effects of co-administrated chromium and carnitine on hormonal parameters. The ages of these participants were 18 to 40 years old. The result revealed a decrease in the level of testosterone hormones57. In addition, a study was conducted on 35 adolescent girls with PCOS, and the participants were provided with six months of chromium supplementation at a strength of 1000 micrograms. The results showed an improvement in the level of free testosterone and decreased menstrual irregularities58.
Chromium and ovarian problem management
In a randomized control trial, chromium picolinate was shown to reduce BMI, fasting insulin levels, and increased the chance of ovulation and a regular menstrual cycle59. In addition, a study was conducted on PCOS females to find the effect of selenium, chromium, myoinositol, and L-tyrosine. The results showed a restoration of ovulation, a regular menstrual cycle, and increased progesterone level in the luteal phase60.
A study was conducted on 60 female females with PCOS to assess the effect of metformin versus chromium picolinate. Results showed that chromium is better tolerated while improving the luteinizing hormone levels, hirsutism score, ovarian aspects, and stimulating follicle hormones61. An increased number of small follicles is present in the ovaries of women with PCOS that show hyperstimulation in the ovaries62. Chromium that reduces the total follicular count in ovaries proves beneficial for treating ovarian problems in women with PCOS. A reduced follicular count also decreases the volume of ovaries58.
Chromium and PCOS management
Insulin resistance is the main feature of polycystic ovarium syndrome, which involves a high serum insulin level, also called hyperinsulinemia. Still, cells are resistant to insulin due to a high frequency of lipid dysfunction63. Chromium is reported to improve insulin resistance and the fasting glucose to insulin ratio, helpful in treating polycystic ovary syndrome59.
Elevated testosterone levels, obesity, and inflammatory markers can increase insulin levels, fasting glucose levels, and cause insulin resistance64. A study conducted on PCOS females concluded that central obesity, despite the normal weight, had an increased risk of causing insulin resistance and dyslipidemia compared with PCOS females of normal weight without central obesity65. Women with polycystic ovaries show abnormal lipid profiles such as high levels of low-density lipoprotein, triglycerides, and low levels of high-density lipoprotein, which affect the overall body composition and play a role in the pathophysiology of PCOS66. A randomized control trial was conducted on 64 women with PCOS, divided into two equal groups of 32 participants. One group was given 200 micrograms of chromium picolinate, and the other group was treated with a placebo for eight weeks. The result showed improvement in most of the clinical features of PCOS, such as the level of serum insulin, serum triglycerides, and cholesterol. Progress in all these parameters also included an improvement of insulin resistance, BMI levels, lipids metabolism, and glucose67, 68. PCOS causes menstrual problems, acne, and hirsutism. The polycystic syndrome causes acne. In both healthy-weight and obese patients, insulin resistance and metabolic syndrome are most common. Many treatments, including weight loss, gonadotropins, metformin, and DASH, are available for patients with PCOS69.
Conclusion
PCOS affects females of reproductive age. It causes disturbances in their menstrual cycle and causes infertility. Acne, fatigue, and hirsutism are common symptoms. Chromium is an important element beneficial for treating polycystic ovaries. It regulates the metabolism of carbohydrates and fats, improves body composition, and decreases BMI. It also governs the disturbances of the hormonal profile due to polycystic ovaries. In addition, benefits include the regulation of the menstrual cycle and an increased chance of ovulation.
Acknowledgments
None.
Author’s contributions
Authors equally contributed to this work. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review board approved the study, and all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

